Method for producing ammonium polyphosphate by utilizing phosphoric acid and urea
A technology of ammonium polyphosphate and urea, applied in chemical instruments and methods, phosphorus compounds, non-metallic elements, etc., can solve the problems of short production process, high raw material requirements, and a large amount of industrial waste, and achieve high polymerization degree and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
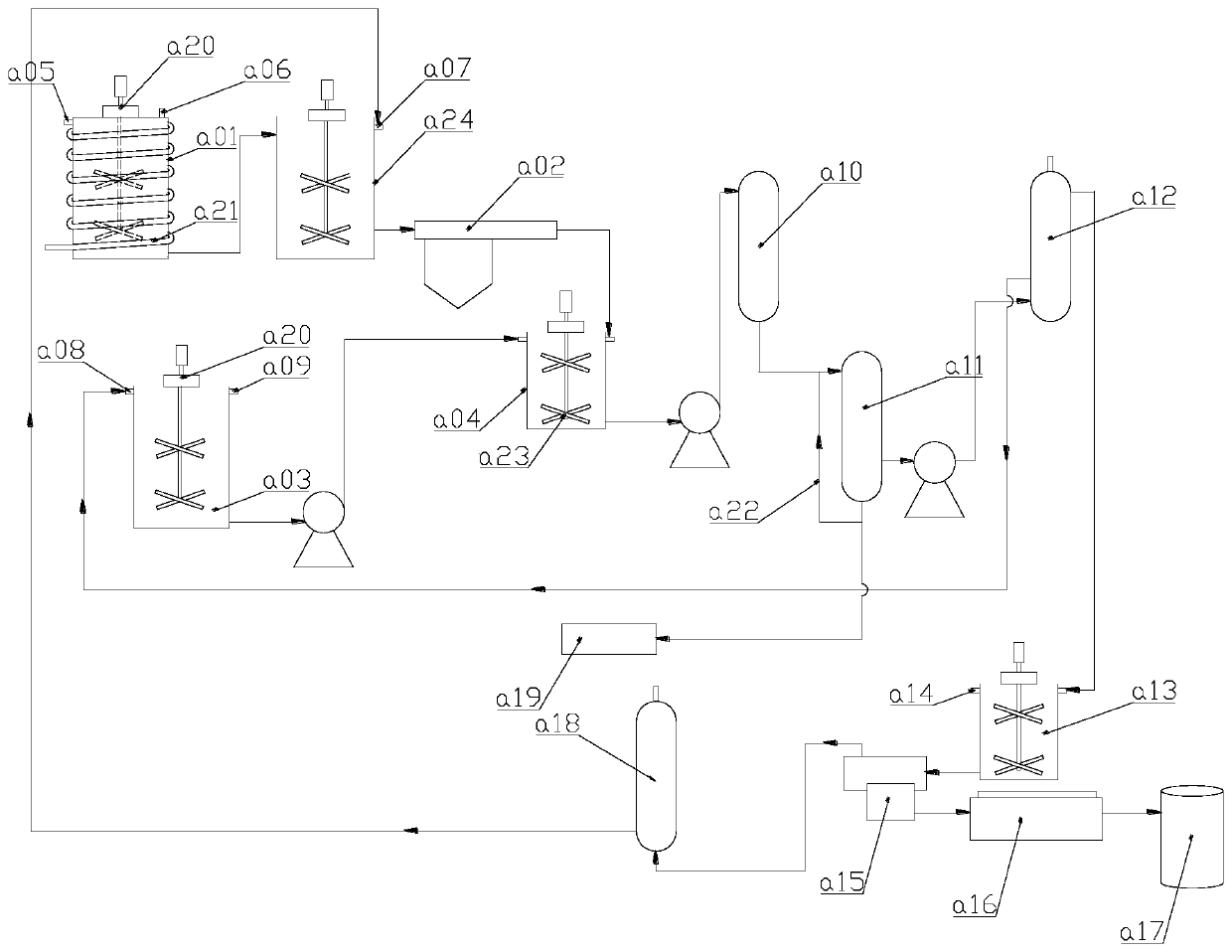
Image
Examples
Embodiment 1
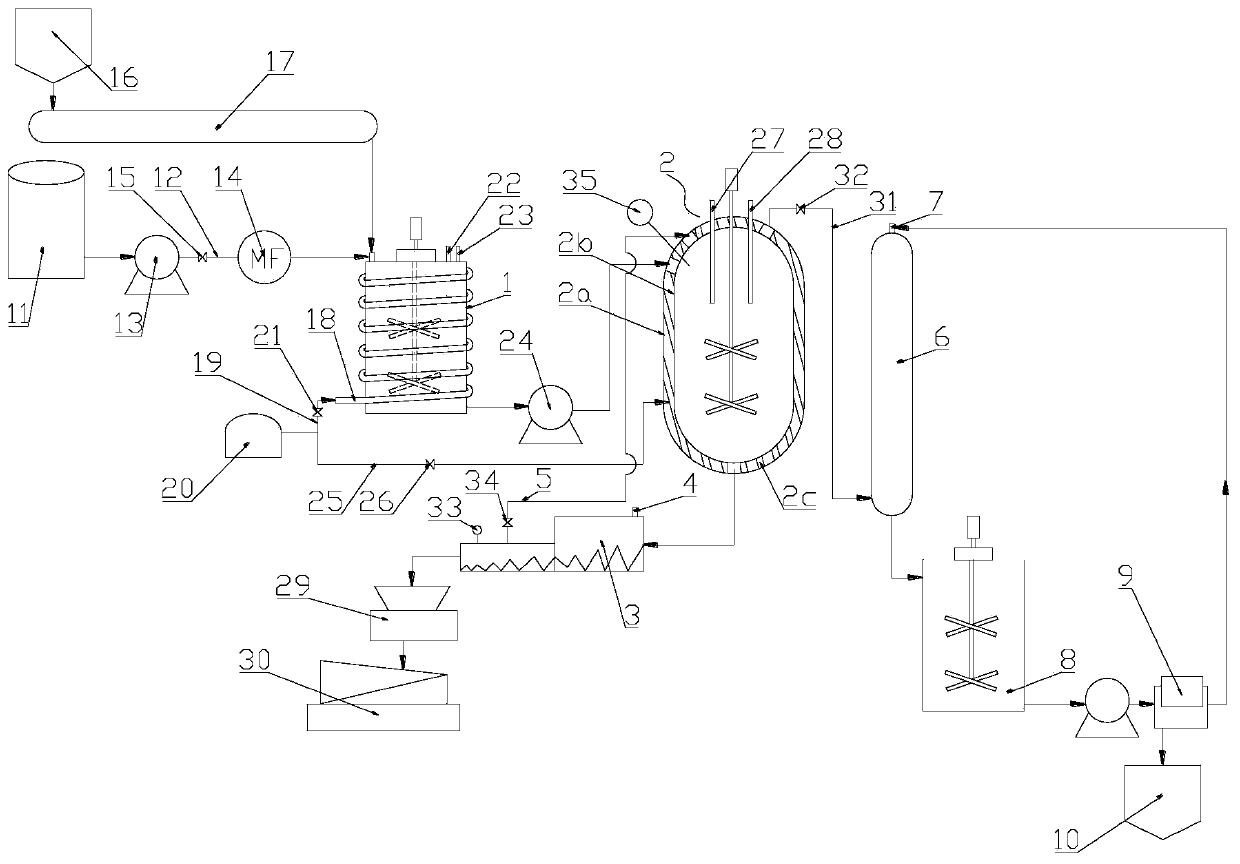
[0029] Example 1, such as figure 1 Shown, a kind of system that utilizes phosphoric acid and urea to produce ammonium polyphosphate comprises a first reaction tank 1, and the feed port of this first reaction tank 1 is connected with phosphoric acid material source and urea material source, and described first reaction tank 1 The discharge port of the reactor is connected with a polymerization kettle 2, a kneader 3, a pulverizer 29 and a screening machine 30 successively, wherein the air inlet of the kneader 3 is connected with an ammonia addition pipe 4, and the polymerization kettle 2 is provided with an ammonia supplement port .
[0030] The phosphoric acid source includes a phosphoric acid tank 11, the outlet of the phosphoric acid tank 11 is connected to the feed port of the first reaction tank 1 through a phosphoric acid feed pipe 12, and the phosphoric acid feed pipe 12 is provided with a first delivery pump 13. The second solenoid valve 15 and the first flowmeter 14. T...
Embodiment 2
[0036] Example 2, such as figure 1 Shown, a kind of method that utilizes the system described in embodiment 1 to produce ammonium polyphosphate, carry out according to the following steps:
[0037] ① Put urea and phosphoric acid into the first reaction tank 1 to react at a molar ratio of 1:0.5, and control the opening and closing of the third solenoid valve 21 through the feedback of the first temperature controller 23, and control the reaction temperature in the first reaction tank to 80 ℃, stirred to form a clear and transparent solution, to obtain a mixed solution of urea phosphate and urea, if the first liquid level controller 22 monitors that the liquid level in the first reaction tank 1 reaches the preset value, the preset value is fed back to the belt metering Weighing 17, stop or slow down the delivery of urea to the first reaction tank 1, while the frequency converter controls the first delivery pump 13 to stop or slow down the delivery of phosphoric acid to the first...
Embodiment 3
[0055] Embodiment 3, a kind of method that utilizes the system described in embodiment 1 to produce ammonium polyphosphate, the difference between this embodiment and embodiment 2 is: the molar ratio of urea and phosphoric acid in step 1. is 1:0.8, and temperature is 100 °C; the first polymerization temperature in step ② is 330 °C for 1.5 hours; the second polymerization time in step ③ is 1 hour.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com