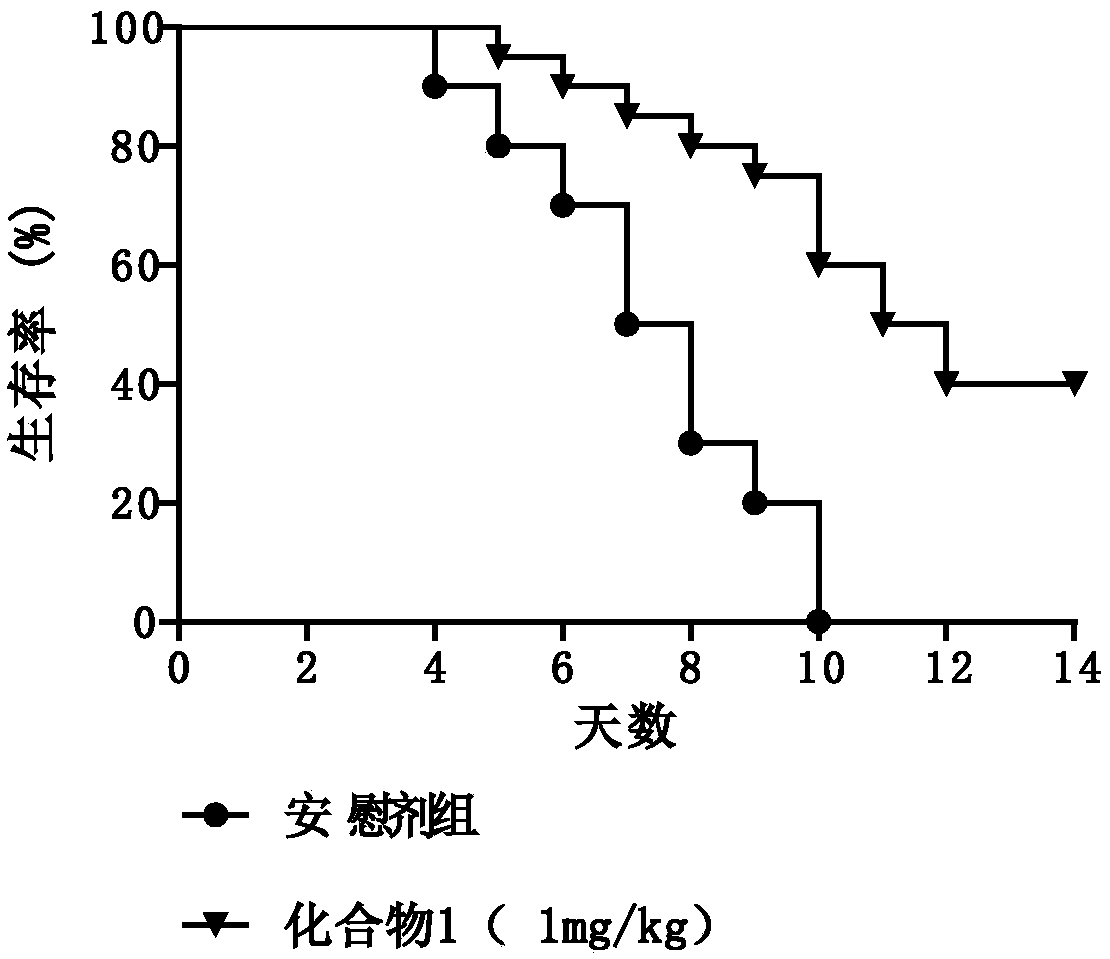
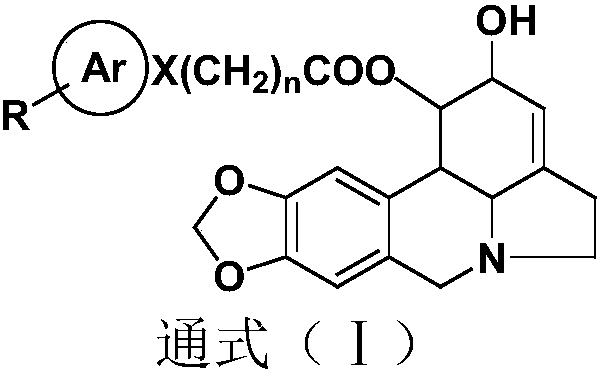
Lycorinine derivative as well as pharmaceutical composition and application thereof
A technology of lycorine and derivatives, which is applied in the field of medicine and can solve problems such as unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: 1-phenoxyacetyl-lycorine (1)
[0110]
[0111] 1-a: Lycorine (1.0mmol), TBSCl (2.0mmol), AgNO 3 (4.0mmol), TEF (4.0mmol), 15ml DMF, placed in a 50ml reaction bottle, stirred at room temperature in the dark until no raw materials remained. Filter, transfer the filtrate to a separatory funnel, add 50ml of dichloromethane, wash the organic phase with saturated sodium bicarbonate solution, water, and saturated sodium chloride solution, dry over anhydrous sodium sulfate, and concentrate to obtain 2-TBS-lycorine .
[0112] 1-b: Put the above-mentioned 2-TBS-lycorine, phenoxyacetic acid (1.0mmol), EDCI (1.2mmol), DMAP (0.1mmol), 15ml of dichloromethane in a 50ml reaction bottle, and stir at room temperature until the raw material Nothing left. Transfer to a separatory funnel, add 50ml of dichloromethane, wash the organic phase with saturated sodium bicarbonate solution, water, and saturated sodium chloride solution, dry over anhydrous sodium sulfate, and conce...
Embodiment 2
[0114] Example 2: 1-Phenylthioacetyl-lycorine (2)
[0115]
[0116] 2-a: Put 2-TBS-lycorine (1.0mmol), phenylthioacetic acid (1.2mmol), EDCI (1.2mmol), DMAP (0.12mmol), 15ml of dichloromethane in a 50ml reaction flask at room temperature Stir until no ingredients remain. Transfer to a separatory funnel, add 50ml of dichloromethane, wash the organic phase with saturated sodium bicarbonate solution, water, and saturated sodium chloride solution, dry over anhydrous sodium sulfate, and concentrate for later use.
[0117] 2-b: The above product, TBAF (2.0mmol), 15ml THF, was placed in a 50ml reaction bottle, and stirred at room temperature until no raw material remained. Transfer to the separatory funnel, add 50ml dichloromethane, wash the organic phase with saturated sodium bicarbonate solution, water, saturated sodium chloride solution respectively, dry over anhydrous sodium sulfate, concentrate, and separate by column chromatography to obtain a yellow solid (32.0% ). 1 H N...
Embodiment 3
[0118] Example 3: 1-(4-Chloro-phenoxyacetyl)-lycorine (3)
[0119]
[0120] 3-a: Put 2-TBS-lycorine (1.0mmol), 4-chloro-phenoxyacetic acid (1.2mmol), EDCI (1.2mmol), DMAP (0.12mmol), 15ml dichloromethane in 50ml for reaction In the bottle, stir at room temperature until no raw material remains. Transfer to a separatory funnel, add 50ml of dichloromethane, wash the organic phase with saturated sodium bicarbonate solution, water, and saturated sodium chloride solution, dry over anhydrous sodium sulfate, and concentrate for later use.
[0121] 3-b: The above product, TBAF (2.0mmol), 15ml THF, was placed in a 50ml reaction bottle, and stirred at room temperature until no raw material remained. Transfer to the separatory funnel, add 50ml dichloromethane, wash the organic phase with saturated sodium bicarbonate solution, water, saturated sodium chloride solution respectively, dry over anhydrous sodium sulfate, concentrate, and separate by column chromatography to obtain a yellow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com