Method for preparing heart progenitor cells
A technology of cardiac progenitor cells and cells, applied in the field of cell biology, can solve the problems of low efficiency of cardiac progenitor cells, difficult molecular cloning experimental operations of large fragment gene sequences, virus packaging, and rapid chromatin remodeling, etc., to achieve improved cell reorganization The effect of programming efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
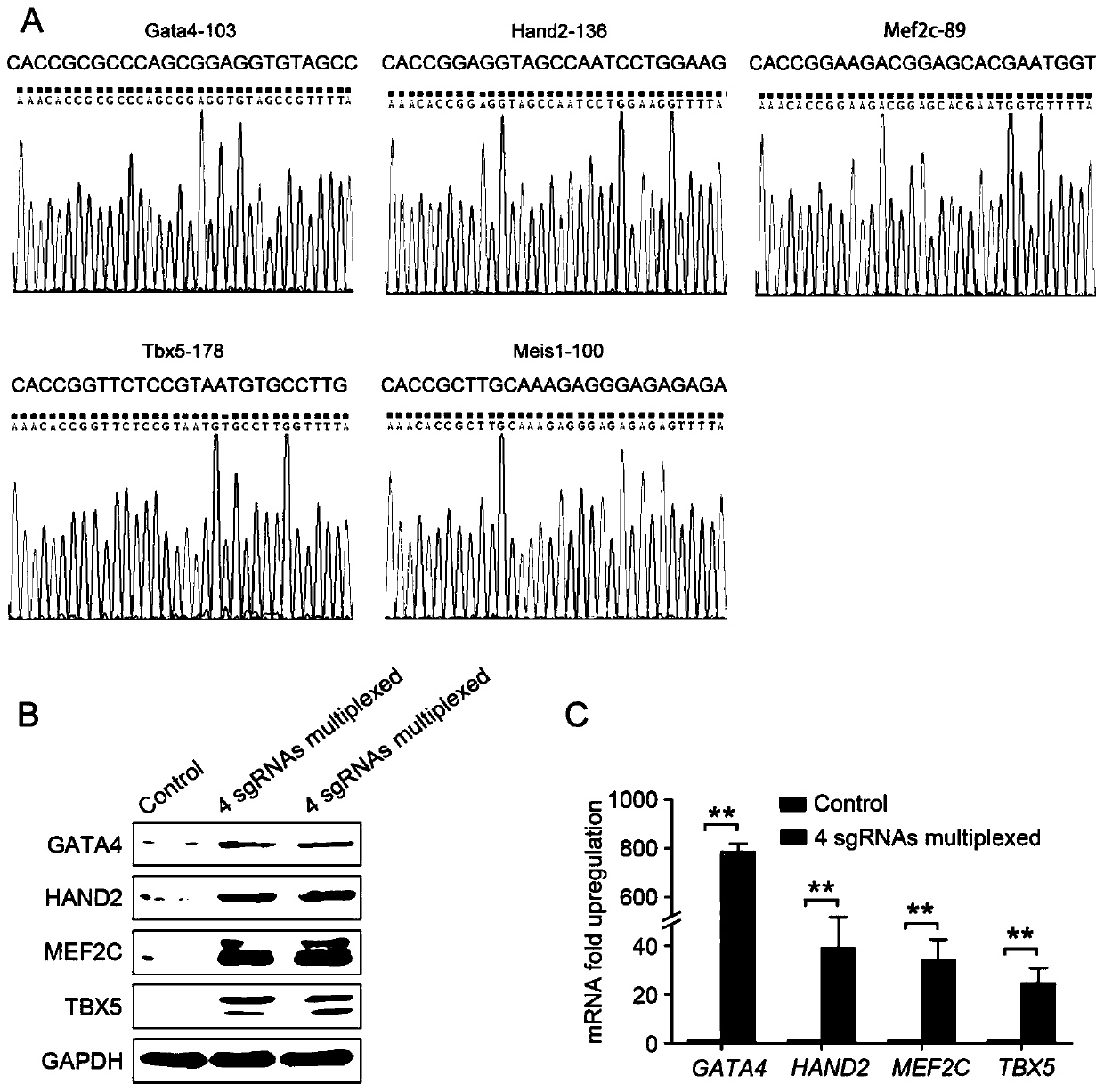
[0087] Example 1 Using the SAM system to induce simultaneous expression of GATA4, HAND2, MEF2C and TBX5 in human foreskin fibroblasts
[0088] (1) Cardiac development-related transcription factors GATA4, HAND2, MEF2C, and TBX5 were selected as reprogramming factors to construct sgRNA-MS2 fusion expression vectors for each gene.
[0089] (2) The day before lentivirus infection of cells, take well-grown human foreskin fibroblasts and inoculate them in a 75cm 2 Cell culture flasks were brought to a cell density of 30-40% at the time of infection.
[0090] (3) Dilute dCas9-VP64 and MS2-p65-HSF1 lentiviral particles at 1:10000 with DMEM medium containing 8 μg / mL polybrene. The cell culture medium was discarded, the cells were gently washed twice with PBS, and 15 mL of DMEM medium containing lentiviral particles was added to infect human foreskin fibroblasts. Human foreskin fibroblasts stably expressing dCas9-VP64 and MS2-p65-HSF1 were screened by Blasticidin S HCL and Hygromycin ...
Embodiment 2
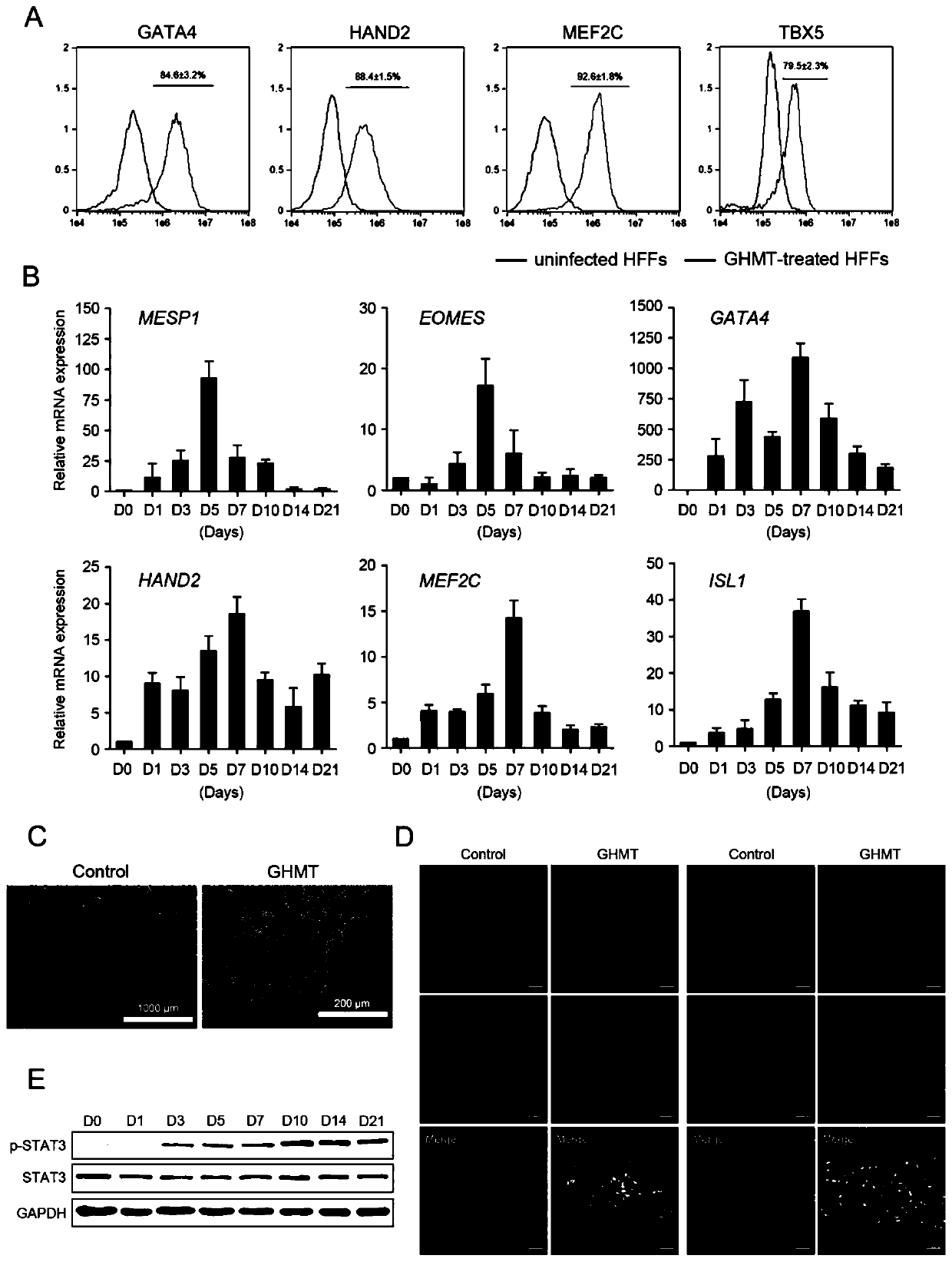
[0096] Example 2: The SAM system induces the formation of cardiac progenitor cells
[0097] (1) Two days before the cells were infected with sgRNA lentiviruses of GATA4, HAND2, MEF2C and TBX5, the DMEM culture medium without Blasticidin S HCL and Hygromycin B was replaced.
[0098] (2) Before cell seeding, pipette 312 μL of Matrigel hESC-qualified Matrix and dilute it in 25 mL of pre-cooled serum-free DMEM / F-12 medium, then take an appropriate amount of diluted Matrigel hESC-qualified Matrix and add them to laser confocal culture dishes and 60 mm culture medium respectively. dish and 100mm petri dish, mix gently, and place at room temperature for 1h.
[0099] (3) Take human foreskin fibroblasts that grow well and stably express dCas9-VP64 and MS2-p65-HSF1 at 4000 cells / cm 2 Inoculated on Matrigel hESC-qualified Matrix-coated Petri dishes.
[0100] (4) Take out the virus liquid from -80°C, put it on ice to dissolve, and then dilute it with DMEM culture medium containing 8 μg / ...
Embodiment 3
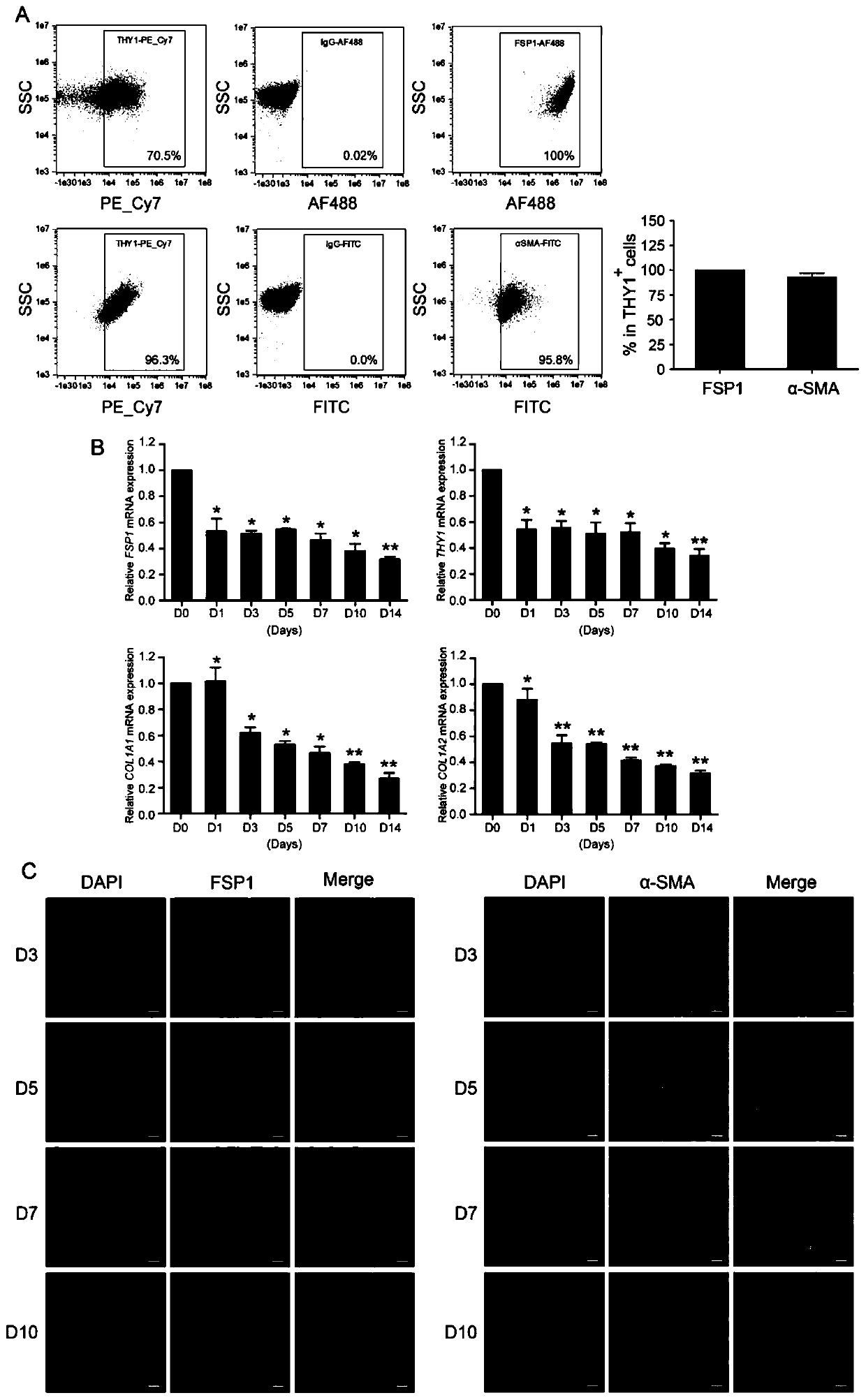
[0109] Example 3: Expression of fibroblast markers gradually down-regulated during cell reprogramming
[0110] (1) First, the expression of fibroblast markers FSP1 and α-SMA was analyzed by flow cytometry.
[0111] (2) Two days before the cells were infected with sgRNA lentiviruses of GATA4, HAND2, MEF2C and TBX5, the DMEM culture medium without Blasticidin S HCL and Hygromycin B was replaced.
[0112] (3) Before cell seeding, pipette 312 μL of Matrigel hESC-qualified Matrix and dilute it in 25 mL of pre-cooled serum-free DMEM / F-12 medium, then take an appropriate amount of diluted Matrigel hESC-qualified Matrix and add them to laser confocal culture dishes and 60 mm culture medium respectively. In a dish, mix gently, and place at room temperature for 1 hour.
[0113] (4) Take human foreskin fibroblasts that grow well and stably express dCas9-VP64 and MS2-p65-HSF1 at 4000 cells / cm 2 Inoculated on Matrigel hESC-qualified Matrix-coated Petri dishes.
[0114] (5) Take out the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com