Electronic quality control card used for time-resolved fluorescent immunoassay instrument and electronic quality control card component
A time-resolved fluorescence and immunological analyzer technology, applied in the field of quality control cards, can solve the problems of electronic quality control card fluorescence intensity attenuation, affecting use and test results, humidity and temperature sensitivity, etc., to shorten preparation time and improve quality control. Accuracy, longevity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
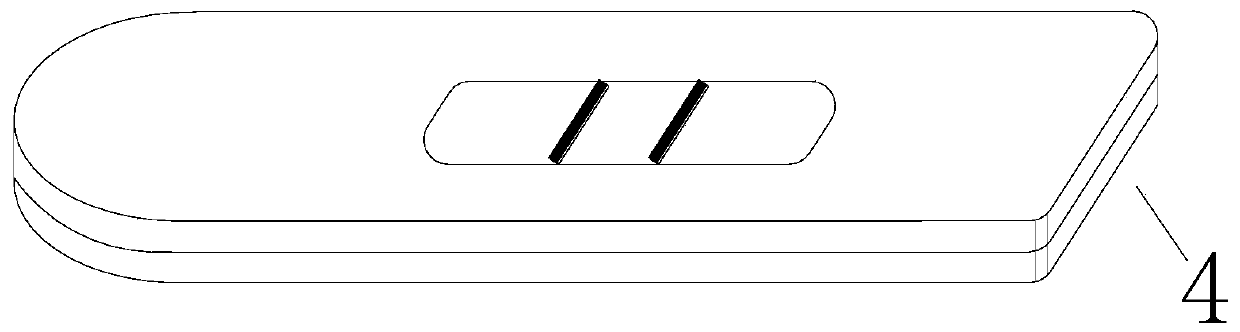
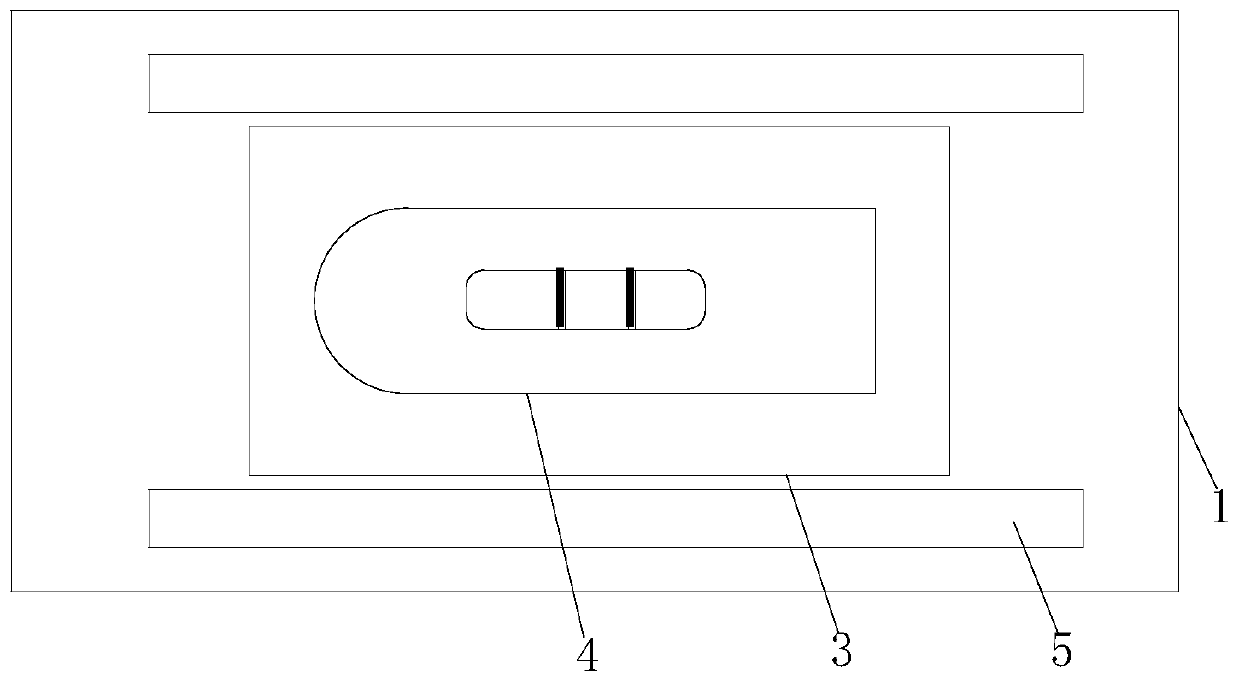
[0045] This embodiment relates to an electronic quality control card for a time-resolved fluorescence immunoassay analyzer, which includes a reagent shell and a quality control NC membrane. The quality control NC membrane carries two fluorescent quality control lines, which are produced through the following steps:
[0046] a. Obtain fluorescent quality control line solution;
[0047] Use 45mmol / L PB solution containing 0.01% (mass percentage) SDS to dilute the carboxyl-modified time-resolved fluorescent microspheres according to the high and low concentrations. After dilution, the fluorescent microspheres have no cross-linking and are in a single state. , to obtain a quality control line solution with high fluorescence intensity and another quality control line solution with low fluorescence intensity; wherein the time-resolved fluorescent microspheres are made of polystyrene;
[0048] b. Draw a fluorescent quality control line on the NC membrane;
[0049] Scribing and dryin...
Embodiment 2
[0069] This embodiment relates to the evaluation of the verification results of the time-resolved fluorescence immunoassay analyzer by the electronic quality control card of the present invention.
Embodiment 21
[0071] This embodiment relates to the comparison between the calibration of the electronic quality control card of the present invention and the conventional calibration of the time-resolved fluorescence immunoassay analyzer.
[0072] The electronic quality control card of the present invention verifies the time-resolved fluorescence immunoassay analyzer operation process:
[0073] When using the quality control card for the first time, the information on the matching RFID card needs to be input to the time-resolved fluorescence analyzer; the quality control card of the present invention is inserted into the analyzer, and the analyzer first recognizes the two-dimensional code on the quality control card, completes the identification and puts it on the analyzer. The parameter data of the quality control card is stored, and the analyzer automatically scans the high fluorescence value and low fluorescence quality control lines on the quality control card, and compares the scanning...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com