Methods of using substituted pyrazole and pyrazole compounds and for treatment of hyperproliferative diseases
A technology for proliferative diseases and compounds, applied in drug combination, organic chemistry, pharmaceutical formulation, etc., can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0347] General synthetic method
[0348] There are many general references available that provide generally known chemical synthesis schemes and conditions that can be used to synthesize the disclosed compounds (see, for example, Smith and March, "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structures (March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure), Fifth Edition, Wiley-Interscience, 2001; or Vogel, A Textbook of Practical Organic Chemistry, Including Qualitative Organic Analysis (ATextbook of Practical Organic Chemistry, Including Qualitative Organic Analysis), Fourth Edition, New York (New York): Longman (Longman, 1978).
[0349] The compounds described herein can be purified by any means known in the art, including chromatography, such as HPLC, preparative thin layer chromatography, flash column chromatography, and ion exchange chromatography. Any suitable stationary phase can be used, including normal phase and reverse phase and i...
example 1
[1153] Example 1: Anti-cancer activity
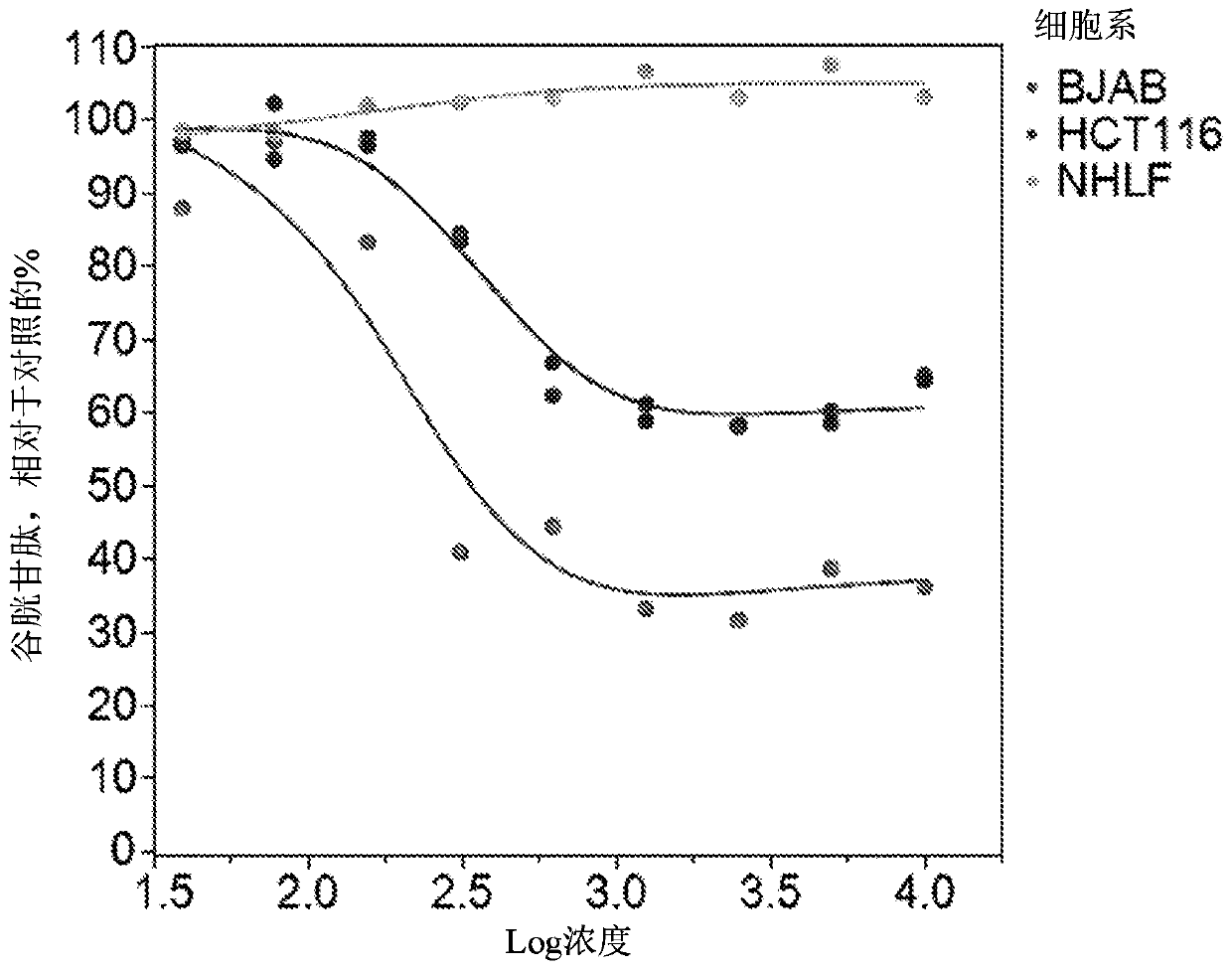
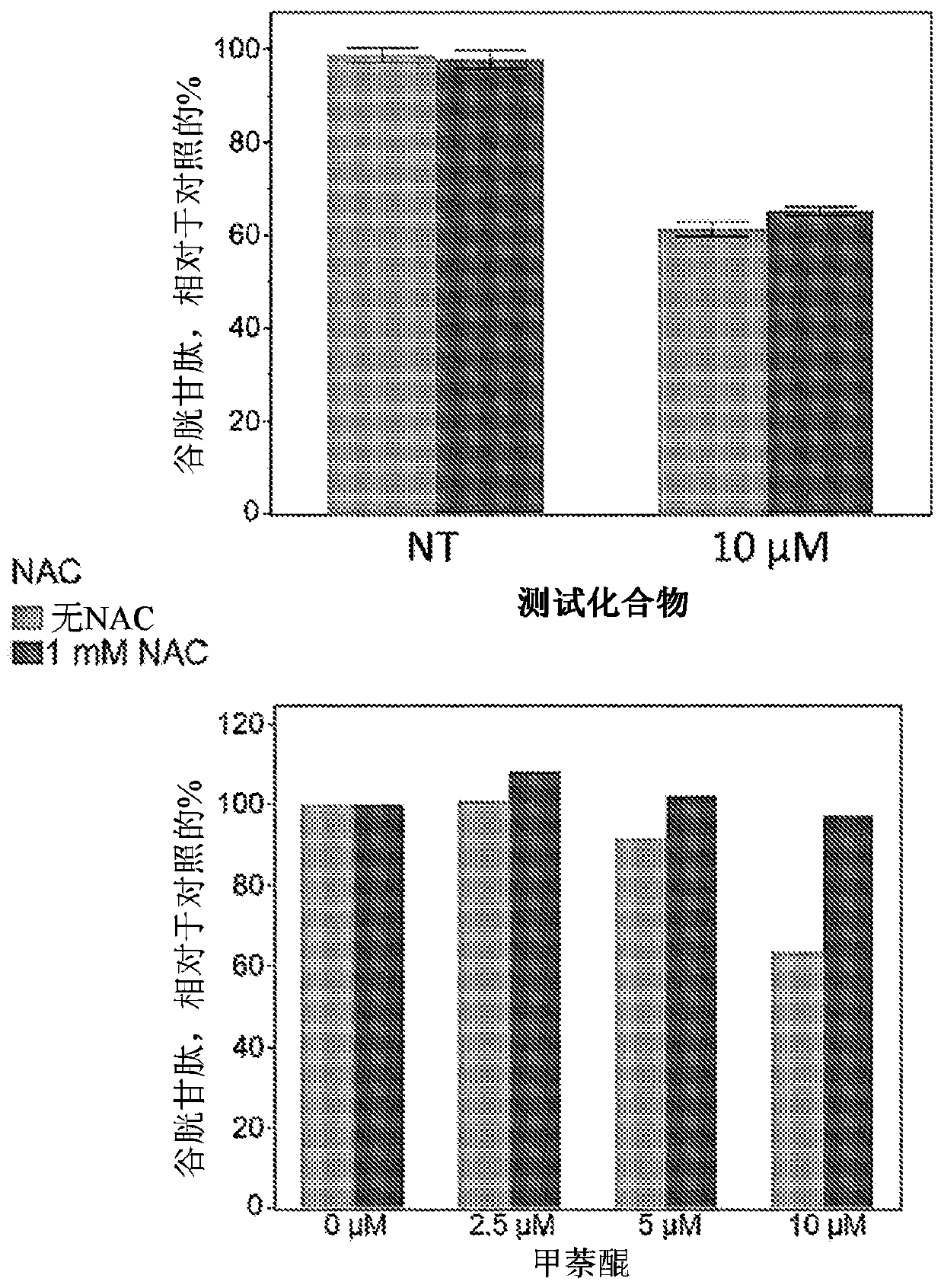
[1154] A group of 96 tumors and 3 normal cell lines were tested for their sensitivity to the test compound. Culture these cell lines in standard medium and pipette them into 96-well plates at the desired plating density. Before compound testing, the cells were allowed to acclimate for 24 hours. Prepare a 20 mM stock solution of the compound in DMSO. To prepare a dose response curve, the compound was serially diluted in DMSO and dispensed into each well of the plate using a Tecan D300e digital dispenser. The final DMSO concentration is 0.15%. After 72 hours of incubation, use (Promega) protocol to determine the number of cells. In this analysis, ATP is measured as a substitute for cell number. The activity of the compound is determined by comparing untreated cells with treated cells and calculating the percentage of signal retained. Compound activity is EC at maximum efficacy level 50 These two values are measured and used to calc...
example 2
[1164] Example 2: The effect of KRAS on activity
[1165] Next, the genotype of the KRAS allele in the solid tumor line was determined. Using the COSMIC database, KRAS genotype and zygosity (homozygous or heterozygous) can be determined for 74 of 77 solid tumor cell lines (Table 3).
[1166] Table 3. Tumor responsiveness regarding KRAS genotype
[1167]
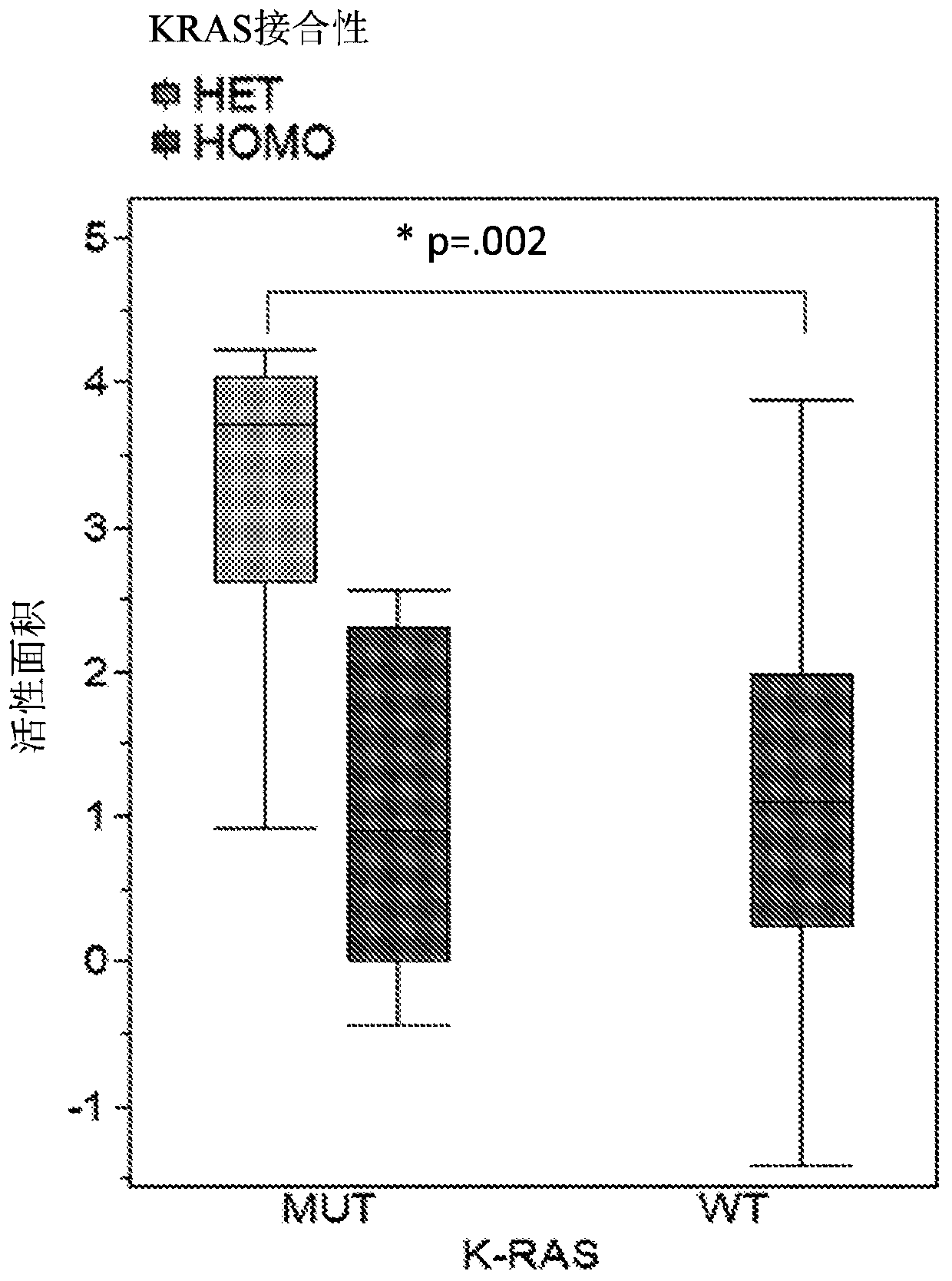
[1168] Only 20% of cell types with wild-type KRAS alleles responded to the test compound. In contrast, 65% of cell types with mutations in the KRAS allele responded to the test compound. When the analysis was extended to whether the KRAS allele was homozygous or heterozygous, the results were significantly different (Table 4). in KRAS Cell lines with heterozygous mutations in alleles showed the greatest reactivity.
[1169] Table 4. Tumor responsiveness regarding KRAS zygosity
[1170]
[1171]
[1172] The final analysis on the role of KRAS in the reaction to the test compounds used compiles data based on the active area of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com