Method of synthesizing diacylglycerol
A technology of diglyceride and partial glyceride, applied in the field of diglyceride synthesis, can solve the problems of weak esterification activity, low esterification activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
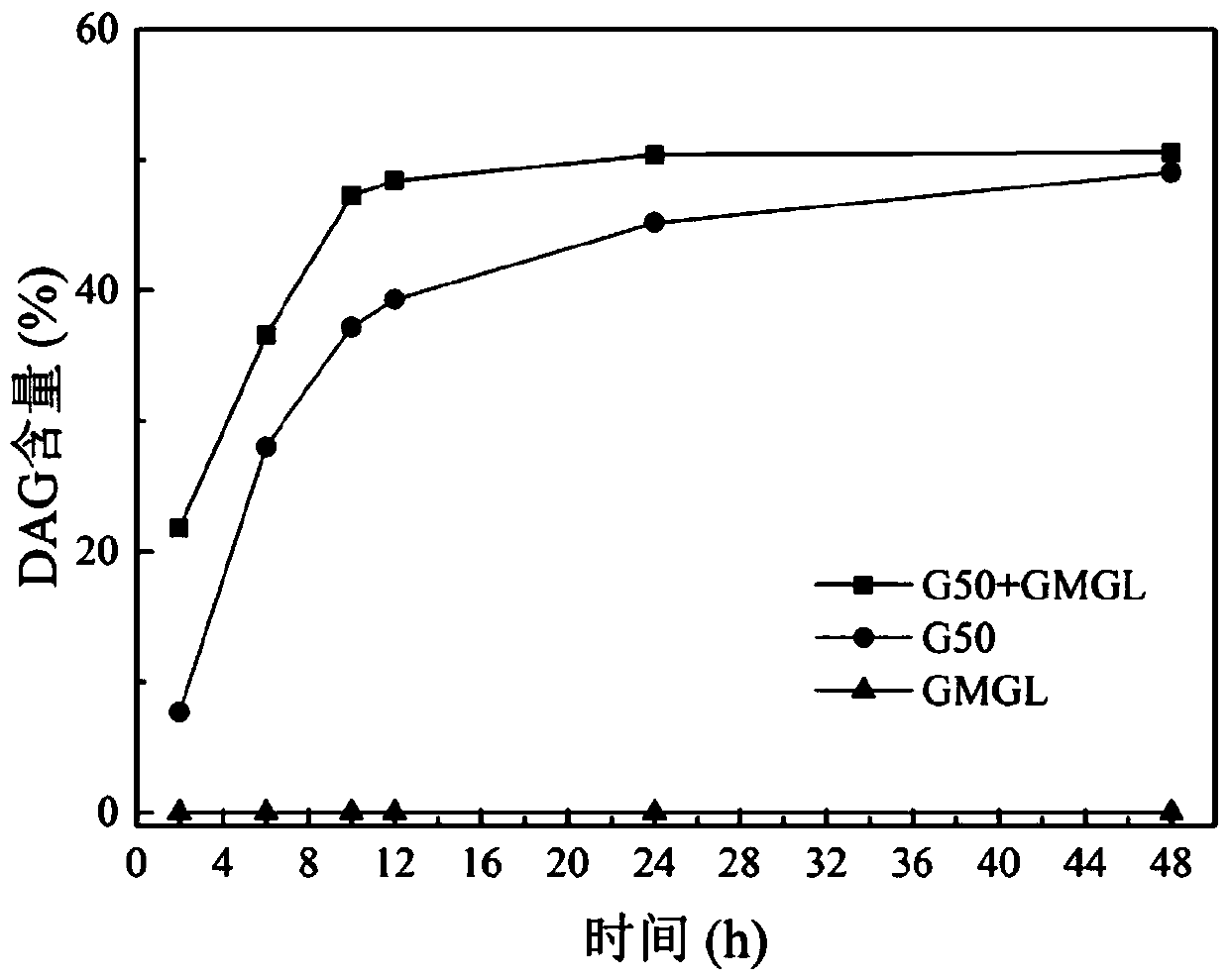
[0023] Example 1G 50 +GMGL
[0024] Get 4.3210g fatty acid and 5.6790g glycerin (molar ratio is 1:4) and the phosphate buffer solution that the pH of 0.4g is 7.5, add in the Erlenmeyer flask with stopper and mix uniformly, and be placed on the constant temperature magnetic stirrer that rotating speed is 500rpm 35 Preheat at ℃ for 10 minutes, add 240U / g partial glyceride lipase Lipase G after the preheating 50 (Based on the total mass of the reactant, purchased from Japan Amano Enzyme Co., Ltd.), while adding 240U / g of monoglyceride lipase GMGL, controlling the reaction temperature to be 35°C, and reacting for 12 hours, the esterification product DAG content was 49.50%. Molecular distillation separation is used for further separation and purification, and the DAG content is as high as 98.07%.
Embodiment 2
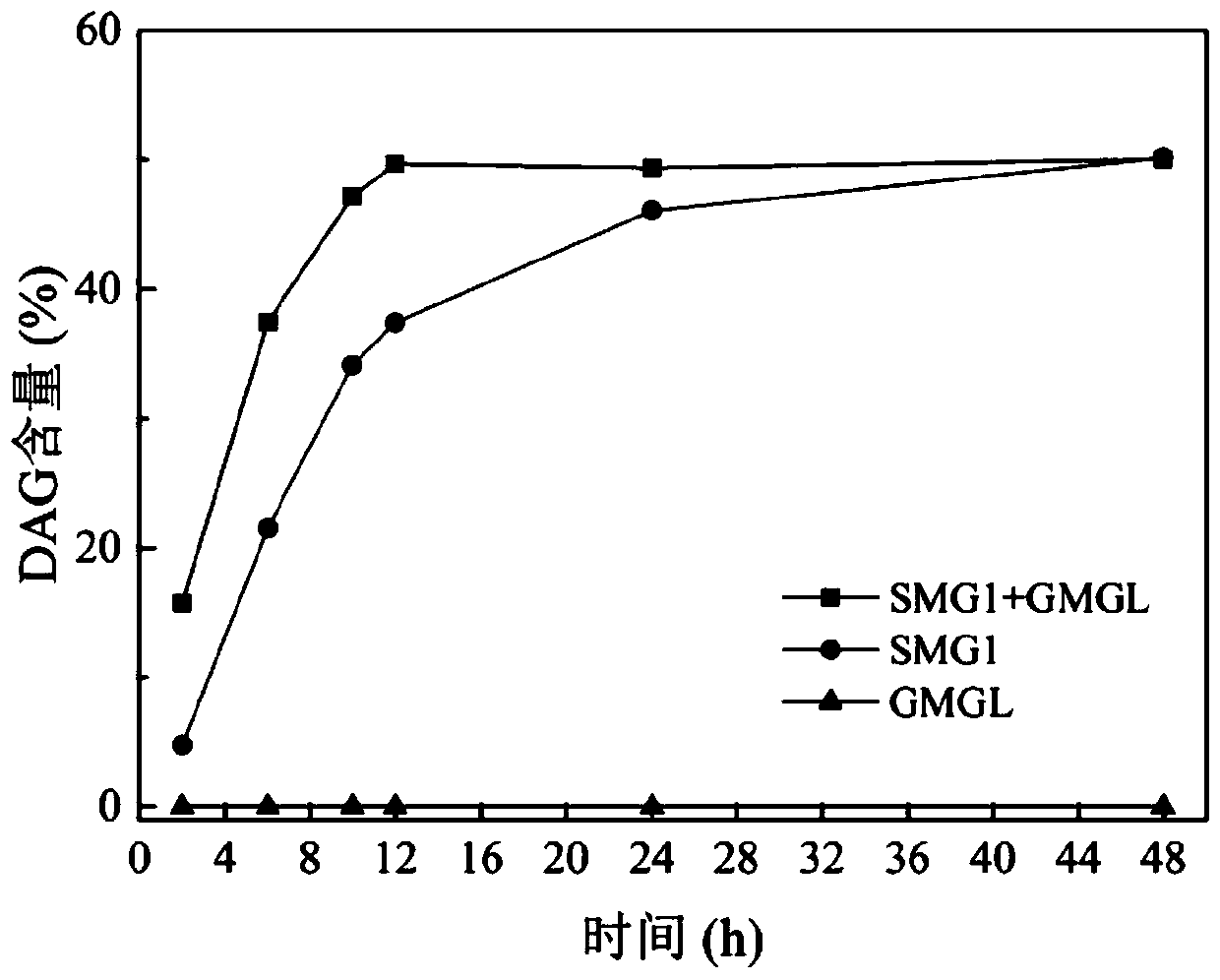
[0025] Example 2 SMG1+GMGL
[0026] Take 4.3210g of fatty acid and 5.6790g of glycerin (molar ratio is 1:4) and 0.4g of pH 7.5 phosphate buffer solution, add it into a stoppered Erlenmeyer flask and mix evenly, and place it on a constant temperature magnetic stirrer with a rotation speed of 500rpm at 35°C Preheat for 10 minutes, add 240U / g partial glyceride lipase SMG1 (based on the total mass of reactants) after preheating, and add 240U / g monoglyceride lipase GMGL at the same time, control the reaction temperature to 35°C; react for 12 hours , the DAG content of the esterification product is 50.04%, and further separated and purified by molecular distillation, the DAG content is as high as 98.30%.
Embodiment 3
[0027] Example 3G 50 +GMGL
[0028] Get 4.3210g fatty acid and 5.6790g glycerol (molar ratio is 1:4) and the pH of 0.2g is the phosphate buffer solution of 7.5, add in the Erlenmeyer flask with stopper and mix uniformly, and be placed on the constant temperature magnetic stirrer that rotating speed is 500rpm 35 Preheat at ℃ for 10 minutes, add 240U / g partial glyceride lipase Lipase G after the preheating 50 (Based on the total mass of reactants), while adding 240U / g of monoglyceride lipase GMGL, controlling the reaction temperature to 35°C, and reacting for 12 hours, the content of DAG in the esterified product was 45.50%, which was further separated and purified by molecular distillation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com