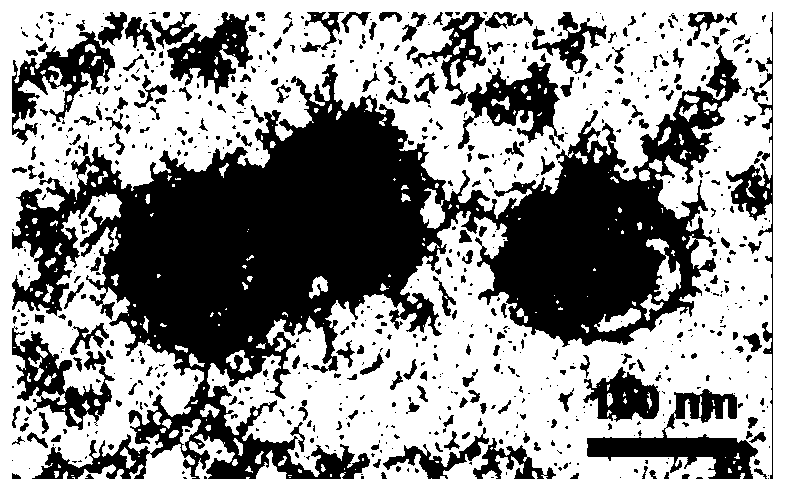
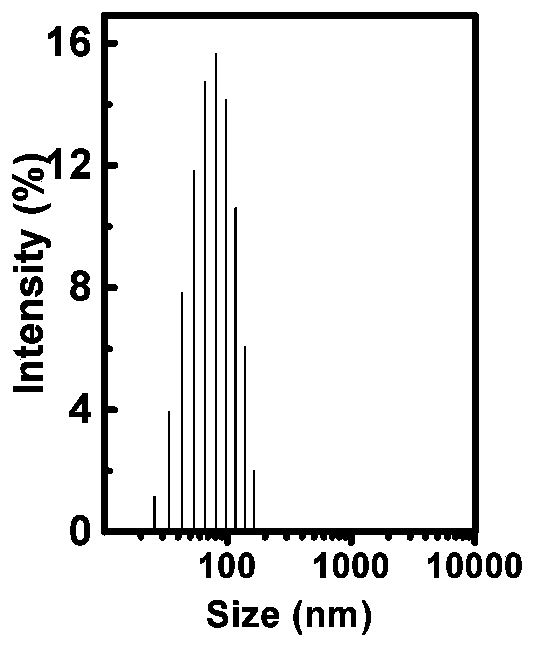
Exosome-based anti-tumor vaccine
An exosome and tumor technology, applied in the field of exosomes, can solve the problems of unsatisfactory tumor inhibition effect and complex tumor microenvironment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention also provides a method for preparing exosomes, which includes the following steps: extracting the nucleus of tumor cells, adding the tumor cell nucleus to the culture medium of antigen-presenting cells, and collecting the supernatant of the culture medium after incubation. centrifuge to collect exosomes.
[0053] In a preferred embodiment of the present invention, the antigen-presenting cells phagocytize tumor cell nuclei to form hybrid cells, and the hybrid cells secrete exosomes, and the exosomes are a type of hybrid exosomes.
[0054] In a preferred embodiment of the present invention, the antigen-presenting cells are dendritic cells, macrophages or B cells, more preferably the antigen-presenting cells are macrophages.
[0055] In a preferred embodiment of the present invention, the endogenous expression of tumor antigens on macrophages is successfully achieved through the strategy of macrophages engulfing tumor cell nuclei. In addition, tumor ...
Embodiment 1
[0073] Example 1, Preparation of E.G7-macrophage hybrid cells
[0074] (a) Cell nuclei derived from lymphoma cell E.G7-OVA (purchased from ATCC cell bank) were extracted using a nucleus extraction reagent (purchased from Solarbio):
[0075] E.G7-OVA cells were cultured in a medium containing 10% fetal bovine serum albumin (purchased from Gibco), 1% penicillin-streptomycin (purchased from Gibco), 0.4mg / mLG418 (purchased from Gibco) and 0.05mMβ- Mercaptoethanol (purchased from Gibco) in high glucose 1640 medium (purchased from Gibco). Subculture once every two days at a ratio of 1:5. The tumor cells in the logarithmic growth phase were collected by centrifugation, resuspended in PBS (Gibco Company), and washed twice. The cell pellet was resuspended using the Lysis buffer in the cell nucleus extraction kit (purchased from Solarbio). After resuspending, the cell density was about 2×10 7 / mL. Afterwards, 20 μL of ReagentA (purchased from Solarbio) was added to each milliliter o...
Embodiment 2、B16
[0079] Example 2, Preparation of B16-macrophage hybrid cells
[0080] (a) Cell nuclei derived from melanoma cells Muc-B16 (purchased from ATCC cell bank) were extracted using a nucleus extraction reagent (purchased from Solarbio):
[0081] Muc1-B16 cells were cultured on the wall, and the complete medium contained fetal bovine serum albumin (purchased from Gibco), 1% penicillin-streptomycin (purchased from Gibco), 1 μg / mL puromycin (purchased from Gibco) 1640 medium (purchased from Gibco, without HEPES), and passaged once every 2-3 days according to the ratio of 1:4. The cells in the logarithmic phase were collected by centrifugation, resuspended in PBS (purchased from Gibco), and washed twice. Will 2×10 7 Each cell was resuspended in Lysis buffer in 1 mL cell nucleus extraction kit (purchased from Solarbio Company), and then 20 μL ReagentA (purchased from Solarbio Company) was added to each milliliter of cell suspension, blown 10 times with a 1 mL pipette tip, and waited W...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com