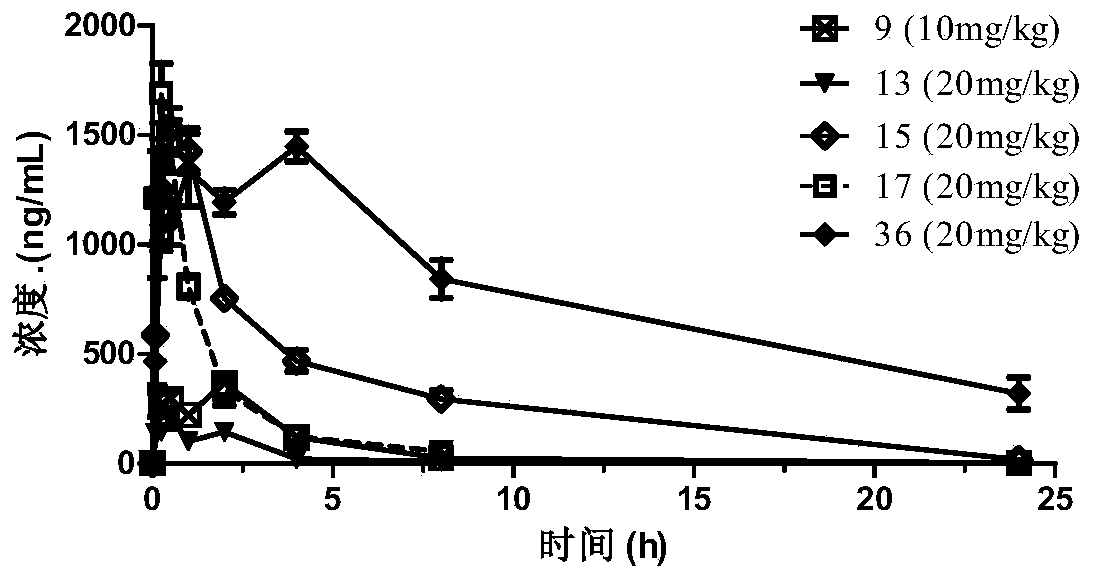
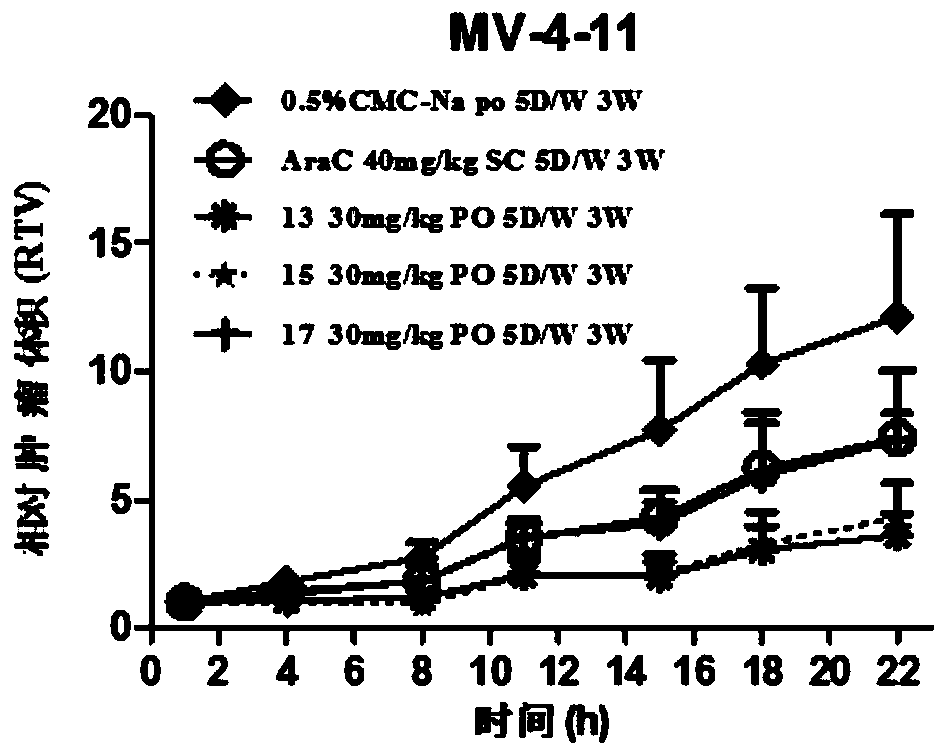
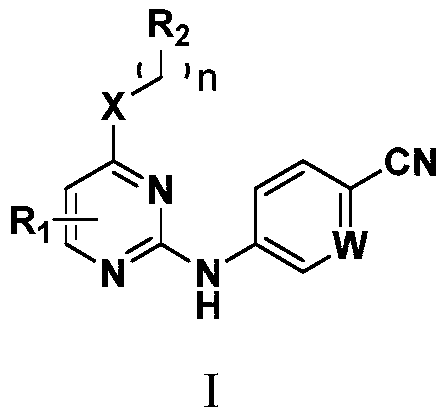
N-subsituted aromatic ring-2-amino pyrimidine compound and applications
An aminopyrimidine and compound technology, applied in the direction of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of reducing the sensitivity of treatment, cell cycle arrest, etc., and achieve the effect of good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0125] Preparation Example 1 5-((4-((piperidin-2-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (Compound 1) synthesis
[0126]
[0127] Step 1. Synthesis of 5-trifluoromethyl-4 chloro-2-aminopyrimidine (intermediate 1-2)
[0128]
[0129] Dissolve 2,4-dichloro-5-trifluoromethylpyrimidine (5.2g, 24.07mmol) in ammonia-saturated ethanol (25ml), stir at room temperature for 2h, recover the solvent under reduced pressure to obtain a residue, and use silica gel column layer Analysis and purification, using PE:EA (5:1) as the eluent, gave white solid 1-2 (2.3g, 11.67mmol), yield: 48.5%. 1 H NMR (500MHz, CDCl 3 )δ8.57(s,1H),7.98(s,2H). ESI-MS:m / z=198[M+H] + .
[0130] Step 2.N 4 -Synthesis of (N-tert-butoxycarbonylpiperidin-2-ylmethyl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (intermediate 1-3)
[0131]
[0132] Under ice-bath condition, take intermediate 1-2 (197.0mg, 1.0mmol), 1-Boc-2-aminomethylpiperidine (256.8mg, 1.2mmol), dissolve in m...
preparation Embodiment 2
[0136] Preparation Example 2 5-((4-((piperidin-3-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (Compound 2)
[0137]
[0138] Step 1.N 4 Synthesis of -(N-tert-butoxycarbonylpiperidin-3-ylmethyl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (intermediate 1-4)
[0139]
[0140] For the synthesis steps, refer to Step 2 of Example 1. Intermediate 1-4 was synthesized by replacing 1-Boc-2-aminomethylpiperidine with 1-Boc-3-aminomethylpiperidine. Yield: 70%. 1 H NMR (500MHz, CDCl 3 )δ8.08(s,1H),5.44(s,1H),5.10(s,2H),3.85(m,2H),3.40(m,2H),3.01(m,1H),2.88–2.73(m ,1H),1.91–1.81(m,2H),1.77(m,2H),1.68
[0141] (m,1H),1.47(s,9H).ESI-MS:m / z=376[M+H] + .
[0142] Step 2. Synthesis of 5-((4-((piperidin-3-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (compound 2)
[0143]
[0144] Synthesis steps Refer to step 3 of Example 1 to synthesize compound 2. Yield: 65%. 1 H NMR(500MHz,DMSO)δ10.30(s,1H),9.07(d,J=2.5Hz,1H),8.42(dd,J=9...
preparation Embodiment 3
[0146] Preparation Example 3 (R)-5-((4-((morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (compound 3)
[0147]
[0148] Step 1. (R)-N 4 Synthesis of -(N-tert-butoxycarbonylmorpholin-2-ylmethyl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (intermediate 1-5)
[0149]
[0150] For the synthesis steps, refer to Step 2 of Example 1. Intermediate 1-5 was synthesized by replacing 1-Boc-2-aminomethylpiperidine with (S)-4-N-Boc-2-aminomethylmorpholine. Yield: 65%. 1 H NMR (500MHz, CDCl 3 )δ8.09(s,1H),5.50(s,1H),5.10(s,2H),4.13–3.67(m,4H),3.66–3.49(m,2H),3.47–3.37(m,1H) ,2.96(s,1H),2.69(s,1H),1.49(s,9H).ESI-MS:m / z=378[M+H] + .
[0151] Step 2. (R)-5-((4-((morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine ( Compound 3) Synthesis
[0152]
[0153] Synthesis steps Referring to Step 3 of Example 1, compound 3 was synthesized. Yield: 70%. 1 H NMR (500MHz, DMSO) δ10.35(s, 1H), 9.04(d, J=2.0Hz, 1H), 8.50–...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com