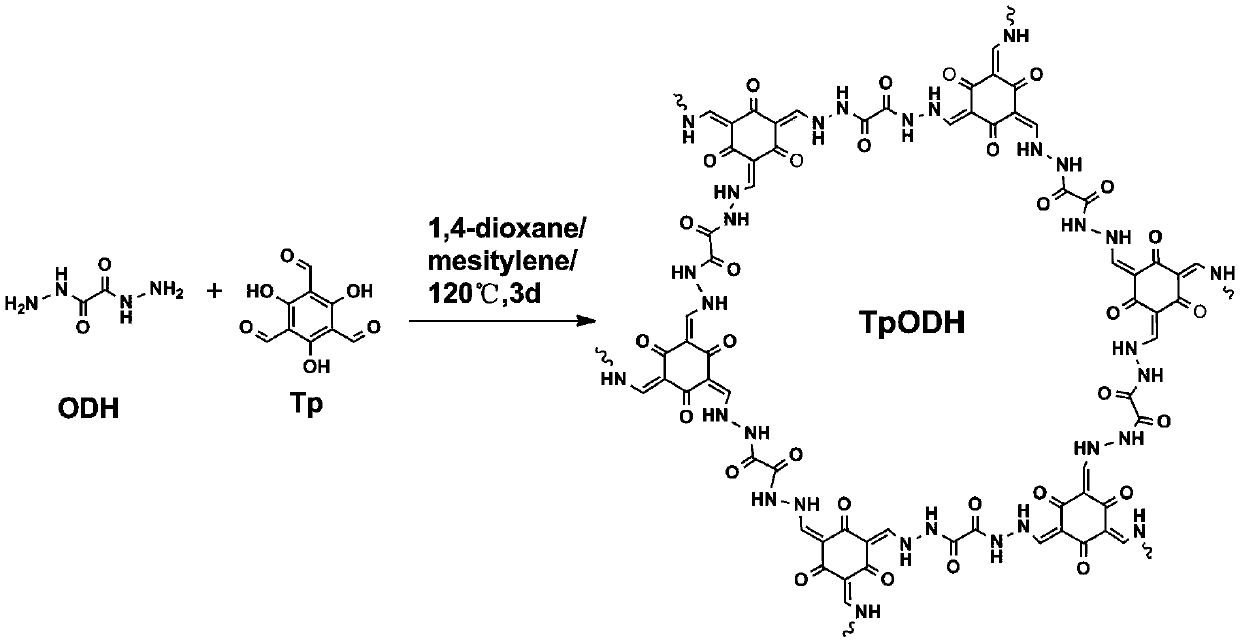
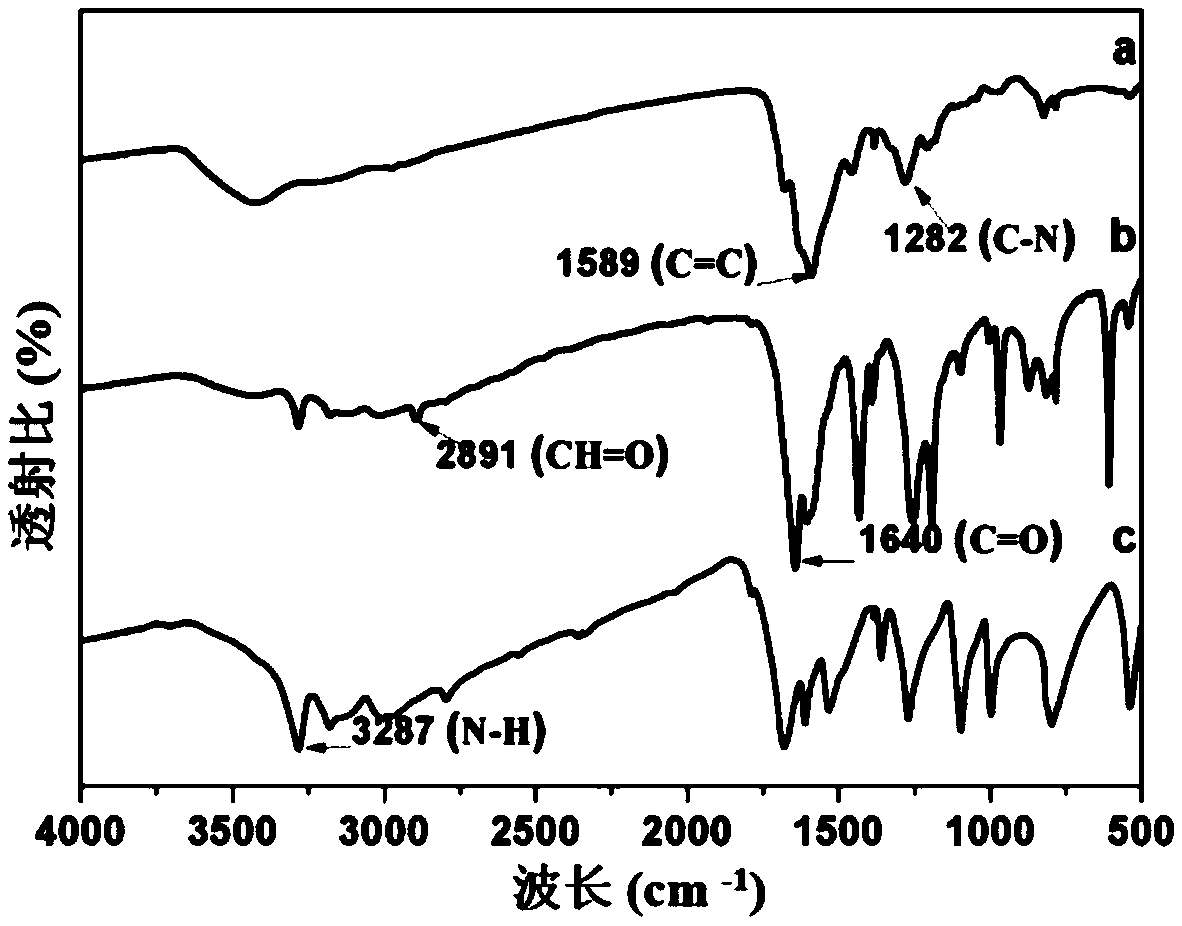
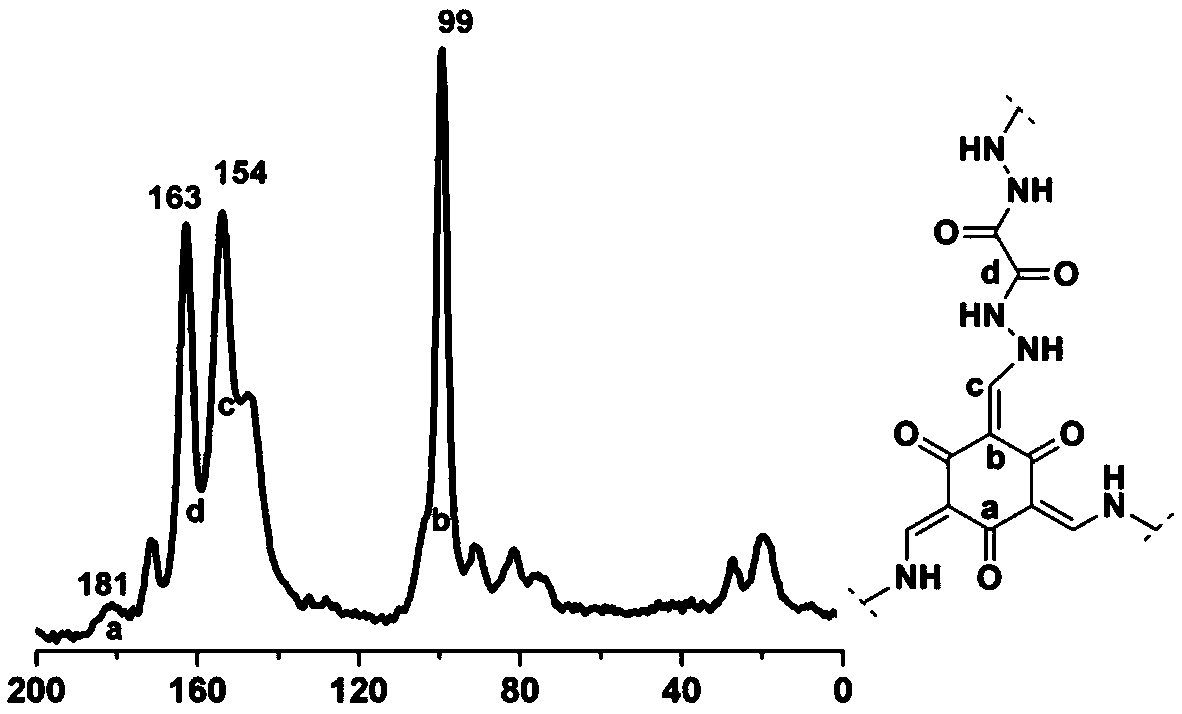
Covalent organic framework material connected through hydrazone bond and preparation method and application thereof
A technology of covalent organic frameworks and covalent organic frameworks, applied in chemical instruments and methods, water/sludge/sewage treatment, water pollutants, etc., can solve problems such as instability and poor chemical stability, and achieve reaction conditions Mild, high specific surface area, green and flexible preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Add 21mg of oxalodihydrazide to a 5mL ampoule.
[0030] 2. Add 17.71 mg of 1,3,5-trialdehyde phloroglucinol to the above centrifuge tube.
[0031] 3. Add 1.5 mL of dioxane, 1.5 mL of mesitylene and 0.6 mL of 6M acetic acid aqueous solution into the above centrifuge tube.
[0032] 4. Sonicate the above-mentioned centrifuge tube for 15 minutes to mix the components in it evenly.
[0033] 5. Seal the mixed solution obtained in step 4 under vacuum.
[0034] 6. Place the sealed ampoule in step 5 in a gas phase furnace at 100°C for 72 hours.
[0035] 7. Wash the material with anhydrous THF and ethanol to remove the reaction solvent and small molecular oligomers.
[0036] 8. Take 10 mg of the COF material obtained in step 7 and add it to a series of 50 mL Erlenmeyer flasks, add 10 mL of heavy metal ion solution (Cu 2+ ,Hg 2+ ,Pb 2+ ,Cr 2+ ,Cd 2+ ), the concentration of each heavy metal ion is 6mmol / L, after adding the COF material, shake at room temperature for 20h....
Embodiment 2
[0039] 1. Add 53mg of oxalodihydrazide to a 5mL ampoule.
[0040] 2. Add 63 mg of 1,3,5-trialdehyde phloroglucinol to the above centrifuge tube.
[0041] 3. Add 1.5mL of o-dichlorobenzene, 1.5mL of N,N-dimethylacetamide and 0.6mL of 6M aqueous acetic acid to the above centrifuge tube.
[0042] 4. Sonicate the above-mentioned centrifuge tube for 15 minutes to mix the components in it evenly.
[0043] 5. Seal the mixed solution obtained in step 4 under vacuum.
[0044] 6. Place the sealed ampoule in step 5 in a gas phase furnace at 100°C for 72 hours.
[0045] 7. Wash the material with anhydrous THF and ethanol to remove the reaction solvent and small molecular oligomers.
[0046] 8. XRD characterization test is to verify that the two monomers do not form a better covalent organic framework structure under the solvent system. Therefore, this solvent system is not suitable for the preparation of COFs with this crystal-ordered structure.
[0047] 9. Take 10 mg of the COF mate...
Embodiment 3
[0049] 1. Add 53mg of oxalodihydrazide to a 5mL ampoule.
[0050] 2. Add 63 mg of 1,3,5-trialdehyde phloroglucinol to the above centrifuge tube.
[0051] 3. Add 3mL of dioxane and 0.6mL of 6M acetic acid aqueous solution to the centrifuge tube.
[0052] 4. Sonicate the above-mentioned centrifuge tube for 15 minutes to mix the components in it evenly.
[0053] 5. Seal the mixed solution obtained in step 4 under vacuum.
[0054] 6. Place the sealed ampoule in step 5 in a gas phase furnace at 100°C for 72 hours.
[0055] 7. Wash the material with anhydrous THF and ethanol to remove the reaction solvent and small molecular oligomers.
[0056] 8. XRD characterization test is to verify that the two monomers do not form a better covalent organic framework structure under the solvent system. Therefore, this solvent system is not suitable for the preparation of COFs with this crystal-ordered structure.
[0057] 9. Take 10 mg of the COF material obtained in step 7 and add it to a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com