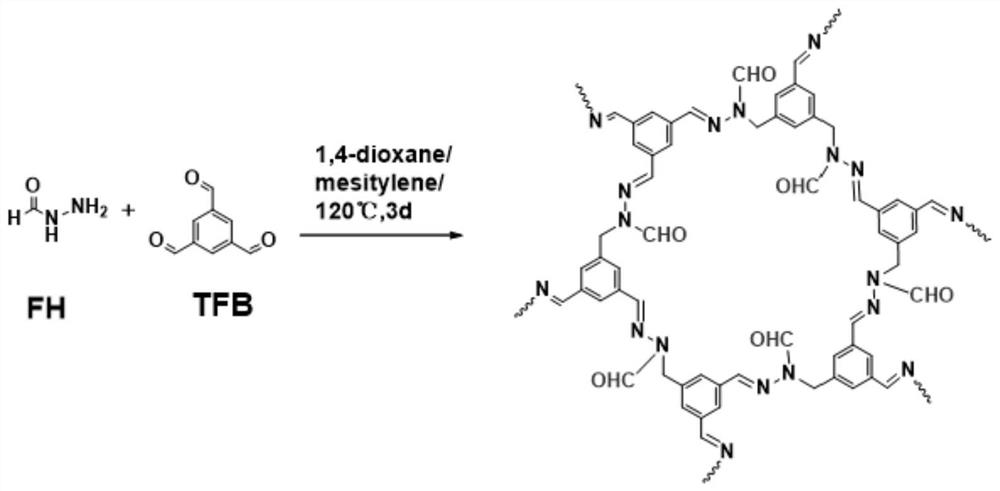
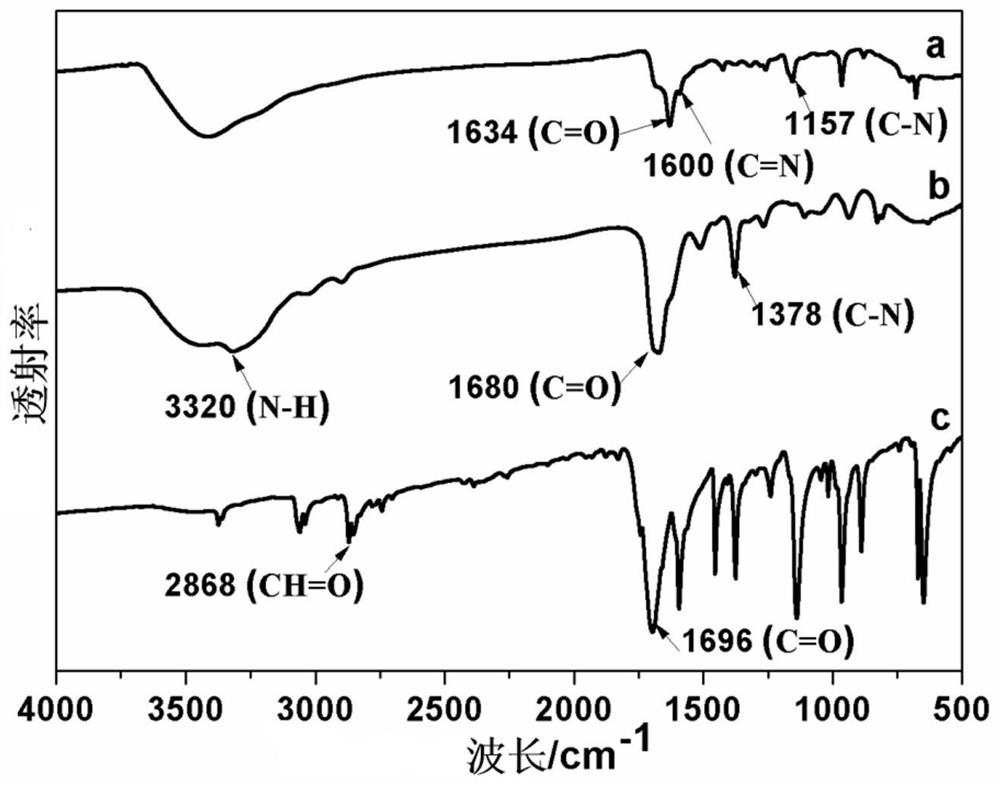
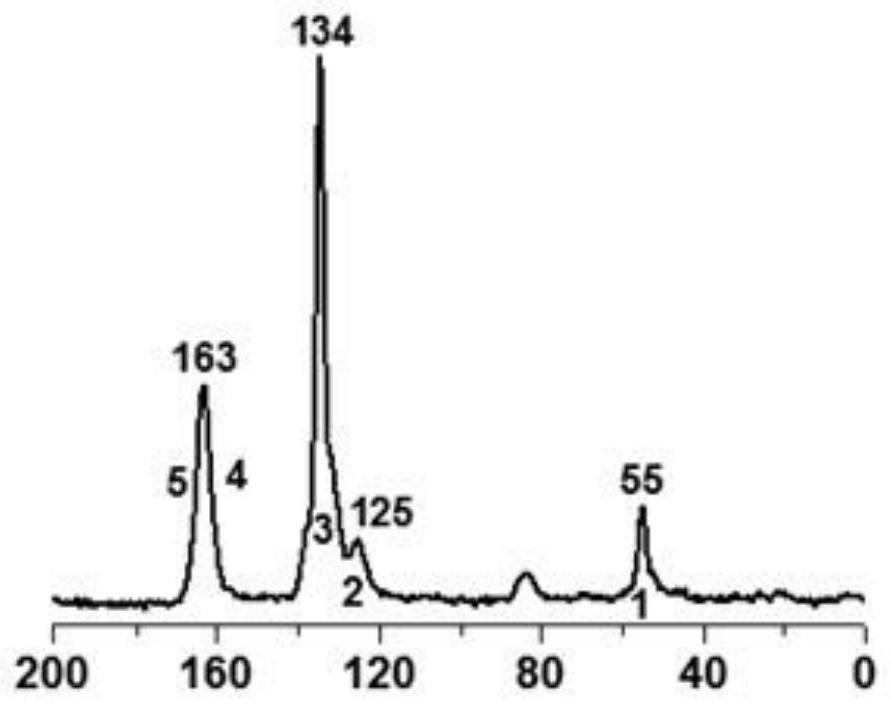
A kind of covalent organic framework material modified by aldehyde group and its preparation and application
A technology of covalent organic framework and aldehyde group modification, applied in other chemical processes, separation methods, products, etc., can solve problems such as COFs morphology research, achieve simple and controllable preparation process, mild reaction conditions, and green and flexible preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Add 9mg of formic hydrazide to a 5mL ampoule.
[0028] 2. Add 16.3 mg of 1,3,5-benzenetricarbaldehyde into the above centrifuge tube.
[0029] 3. Add 1.5 mL of dioxane, 1.5 mL of mesitylene and 0.3 mL of acetic acid water (6M) solution into the above centrifuge tube.
[0030] 4. Sonicate the above-mentioned centrifuge tube for 15 minutes to mix the components in it evenly.
[0031] 5. Seal the mixed solution obtained in step 4 under vacuum.
[0032] 6. Put the sealed ampoule in step 5 in a gas phase furnace at 100°C for 72 hours.
[0033] 7. Wash the material with N,N-dimethylformamide and anhydrous tetrahydrofuran to remove the reaction solvent and small molecule oligomers.
[0034] 8. Take 80mg of the COF material obtained in step 7, and absorb CO under the condition of 298k 2 gas, calculate COF material versus CO 2 The adsorption capacity is 1.6mmol / g.
Embodiment 2
[0036] 1. Add 9mg of formic hydrazide to a 5mL ampoule.
[0037] 2. Add 16.3 mg of 1,3,5-benzenetricarbaldehyde into the above centrifuge tube.
[0038] 3. Add 3 mL of dimethyl sulfoxide and 0.3 mL of acetic acid water (6M) solution into the above centrifuge tube.
[0039] 4. Sonicate the above-mentioned centrifuge tube for 15 minutes to mix the components in it evenly.
[0040] 5. Seal the mixed solution obtained in step 4 under vacuum.
[0041] 6. Put the sealed ampoule in step 5 in a gas phase furnace at 100°C for 72 hours.
[0042] 7. Wash the material with N,N-dimethylformamide and anhydrous tetrahydrofuran to remove the reaction solvent and small molecule oligomers.
[0043] 8. XRD characterization test to verify that the two monomers did not form a better covalent organic framework and hollow tubular structure in this solvent system. Therefore, this solvent system is not suitable for the preparation of COFs with this crystal-ordered structure.
Embodiment 3
[0045] 1. Add 9mg of formic hydrazide to a 5mL ampoule.
[0046] 2. Add 16.3 mg of 1,3,5-benzenetricarbaldehyde into the above centrifuge tube.
[0047] 3. Add 3mL o-dichlorobenzene and 0.3mL acetic acid water (6M) solution to the above centrifuge tube.
[0048] 4. Sonicate the above-mentioned centrifuge tube for 15 minutes to mix the components in it evenly.
[0049] 5. Seal the mixed solution obtained in step 4 under vacuum.
[0050] 6. Put the sealed ampoule in step 5 in a gas phase furnace at 100°C for 72 hours.
[0051] 7. Wash the material with N,N-dimethylformamide and anhydrous tetrahydrofuran to remove the reaction solvent and small molecule oligomers.
[0052] 8. XRD characterization test to verify that the two monomers did not form a better covalent organic framework and hollow tubular structure in this solvent system. Therefore, this solvent system is not suitable for the preparation of COFs with this crystal-ordered structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com