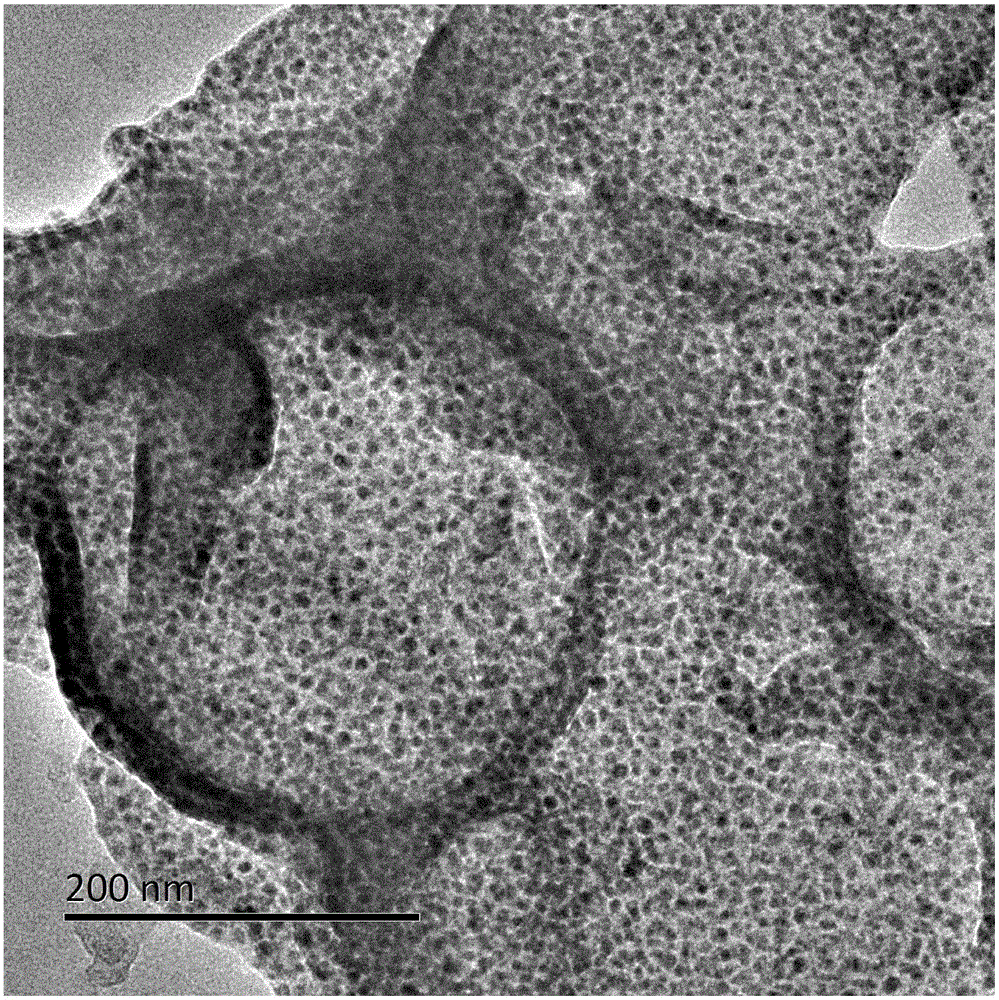
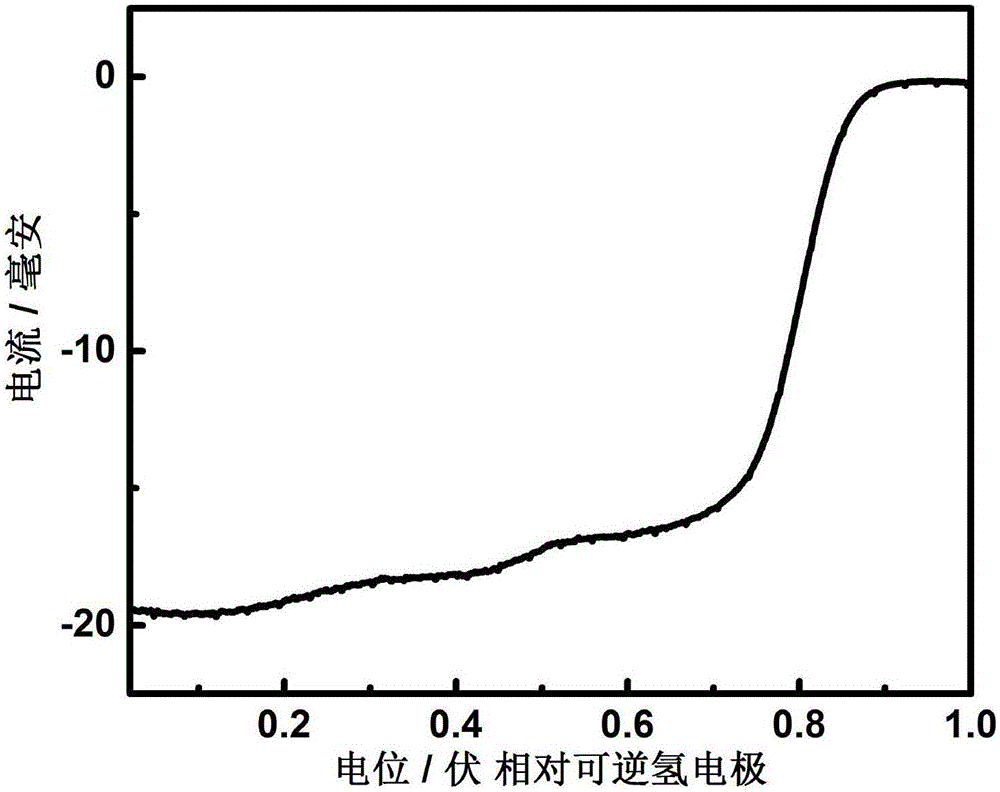
Nitrogen-doped porous carbon nanosheet-supported non-noble metal catalyst and preparation method thereof
A nitrogen-doped porous carbon, non-precious metal technology, used in chemical instruments and methods, physical/chemical process catalysts, nanotechnology, etc. Synthetic, good for repetition and environmental protection, wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Add 0.25 mol of vinylimidazole to a single-necked flask, then add 0.25 mol of nitric acid (concentration: 1 mol / L), stir at room temperature for 2 hours, then raise the temperature to 50°C, and stir for 12 hours to obtain a viscous liquid.
[0038] (2) Add 2.5 mol of cobalt nitrate to the ethanol solution of the viscous liquid above, stir at 70°C, react for 2 hours, and dry at 50°C to obtain a purple solid powder.
[0039] (3) Put the above solid powder into a porcelain boat, calcinate in a tube furnace, use N2 as protective gas, flow rate is 10mL / min, heat up to 100°C at a rate of 5°C / min, keep warm for 1 hour, and then The temperature was raised to 650°C at a rate of ℃ / min for calcination for 2 hours, and the product was naturally cooled to room temperature to obtain a black fluffy solid product, and the nitrogen-doped carbon nanosheet-supported Co catalyst was obtained after the product was ground.
Embodiment 2
[0041] (1) Add 0.1 mol of propenylimidazole to a one-necked flask, then add 1 mol of nitric acid, stir at room temperature for 2 hours, then raise the temperature to 60°C, stir for 15 hours, and obtain a viscous liquid.
[0042] (2) Add 0.05 mol of cobalt nitrate to the above viscous liquid, stir at 70° C., and react for 1 hour to obtain a purple solid powder.
[0043] (3) Put the above-mentioned solid powder into a porcelain boat, calcinate in a tube furnace, use N2 as protective gas, flow rate is 25mL / min, heat up to 100°C at a rate of 5°C / min, keep warm for 1 hour, and then The temperature was raised to 800°C at a rate of °C / min, kept at a temperature of 2 hours, and naturally cooled to room temperature to obtain a black fluffy solid, which was ground to obtain a catalyst powder.
Embodiment 3
[0045] (1) Add 1mol 1-butenylimidazole to a single-necked flask, then add 0.5mol nitric acid (concentration: 0.5mol / L), stir at room temperature for 2 hours, then heat up to 50°C and stir for 12 hours to obtain a viscous liquid .
[0046] (2) Add 0.05 mol of ferric nitrate to the above viscous liquid, stir at 70° C., and react for 2 hours to obtain solid powder.
[0047] (3) Put the above solid powder into a porcelain boat, calcinate in a tube furnace, use N2 as a protective gas, raise the temperature to 100 degrees at 5°C / min, keep it warm for 1 hour, then raise the temperature to 900°C at 5°C / min, and keep it warm After two hours, the temperature was naturally lowered to obtain a black fluffy solid, which was ground to obtain a catalyst powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com