Florfenicol intermediate state compound, and method for preparing florfenicol intermediate
A technology of florfenicol and intermediates, which is applied in the field of steroid hormone preparation and can solve problems such as hidden dangers of safety, release of dichloromethane, and harm to the environment of operators.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Such as Figure 4 Said microchannel reaction system includes a batching tank 1, a constant flow pump 2, a microchannel reactor 3, a back pressure valve 4 and a receiving tank 5 connected in sequence.
[0028] 30kg of dichloromethane and 3.1kg of diethylamine are pumped into batching kettle 1, and 7kg of hexafluoropropylene is passed through after cooling down to below -30°C. Stir for 30 minutes, add (4R, 5R)-2-dichloromethyl-4-hydroxymethyl-4,5-dihydro-5-(4-(methylsulfonyl)phenyl)oxazoline 10kg, Stir at room temperature for 1 hour to dissolve and obtain a solution, which contains the intermediate compound of Florfenicol.
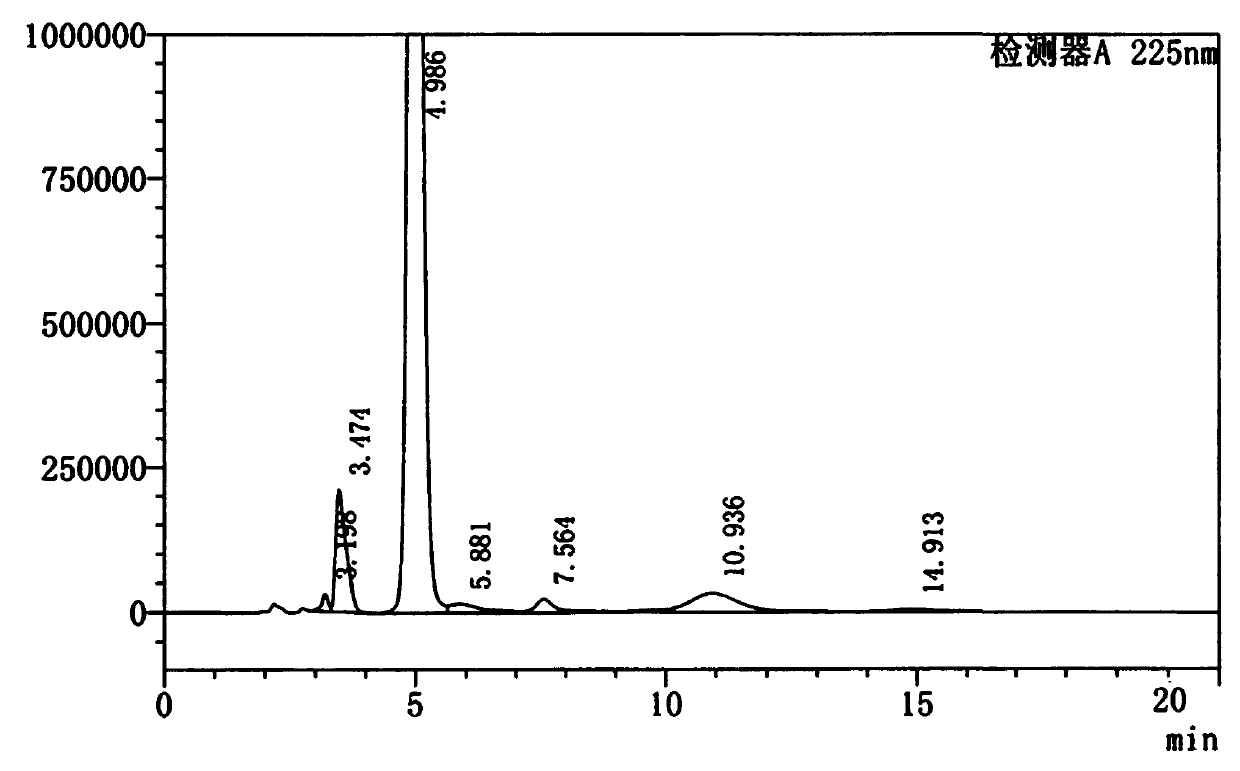
[0029] Figure 5-6 As the raw material, the HPLC spectrum of (4R, 5R)-2-dichloromethyl-4-hydroxymethyl-4,5-dihydro-5-(4-(methylsulfonyl)phenyl)oxazoline and peak analysis tables.
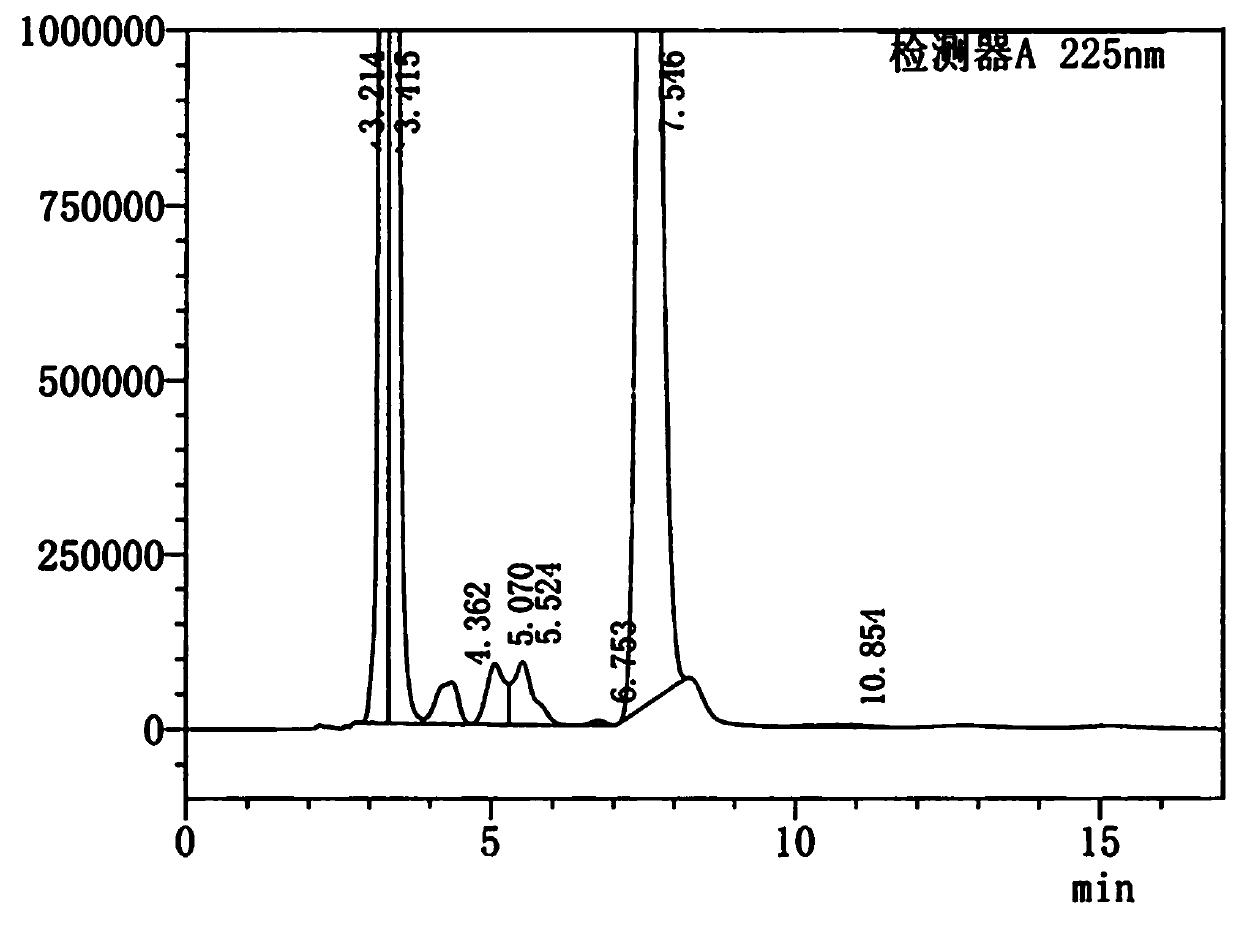
[0030] Figure 3-4 It is the HPLC spectrum and peak analysis table of the intermediate state compound of Florfenicol.
[0031] In the above solution, the purity of th...
Embodiment 2
[0037] 30kg of dichloromethane and 3.1kg of diethylamine were pumped into the batching kettle, and 7kg of hexafluoropropylene was passed through after cooling down to below -30°C. Stir for 30 minutes, add (4R, 5R)-2-dichloromethyl-4-hydroxymethyl-4,5-dihydro-5-(4-(methylsulfonyl)phenyl)oxazoline 10kg, Stir at room temperature for 1 hour.
[0038] Adjust the pressure of the back pressure valve 4 to 1.7MPa, and the temperature of the external bath rises to 140°C. After the temperature is constant, start the constant flow pump 2, and pump the mixture in the batching kettle 1 into the microchannel reactor 3 at a flow rate of 100ml / min. Sampling and testing, the reaction is complete, the reaction solution in the receiving kettle 5 is concentrated to dryness under reduced pressure, 30 kg of isopropanol, 40 kg of water, and 4.5 kg of anhydrous sodium acetate are added, and the sample is tested after reflux reaction for 8 hours. Isopropanol, cooled to 20-25° C., centrifuged, and drie...
Embodiment 3
[0040] 30kg of dichloromethane and 3.1kg of diethylamine were pumped into the batching kettle, and 7kg of hexafluoropropylene was passed through after cooling down to below -30°C. Stir for 30 minutes, add (4R, 5R)-2-dichloromethyl-4-hydroxymethyl-4,5-dihydro-5-(4-(methylsulfonyl)phenyl)oxazoline 10kg, Stir at room temperature for 1 hour.
[0041] Adjust the pressure of the back pressure valve to 1.8MPa, and the temperature of the external bath rises to 150°C. After the temperature is constant, start the constant flow pump, pump the mixture in the batching kettle into the microchannel reactor at a flow rate of 150ml / min, and take samples from the effluent for detection. After the reaction is complete, concentrate the reaction solution in the receiving kettle to dryness under reduced pressure, add 30kg of isopropanol, 40kg of water, and 4.5kg of anhydrous sodium acetate, and take a sample for 8 hours of reflux reaction. to 20–25°C, centrifuge, and dry to obtain 10.23kg of florf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com