Anti-CD47/VEGF bispecific antibody and application thereof
A bifunctional antibody, antibody technology, applied in the field of tumor immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Example 1 Molecular Sequence Design of Anti-CD47 / VEGF Bispecific Antibody
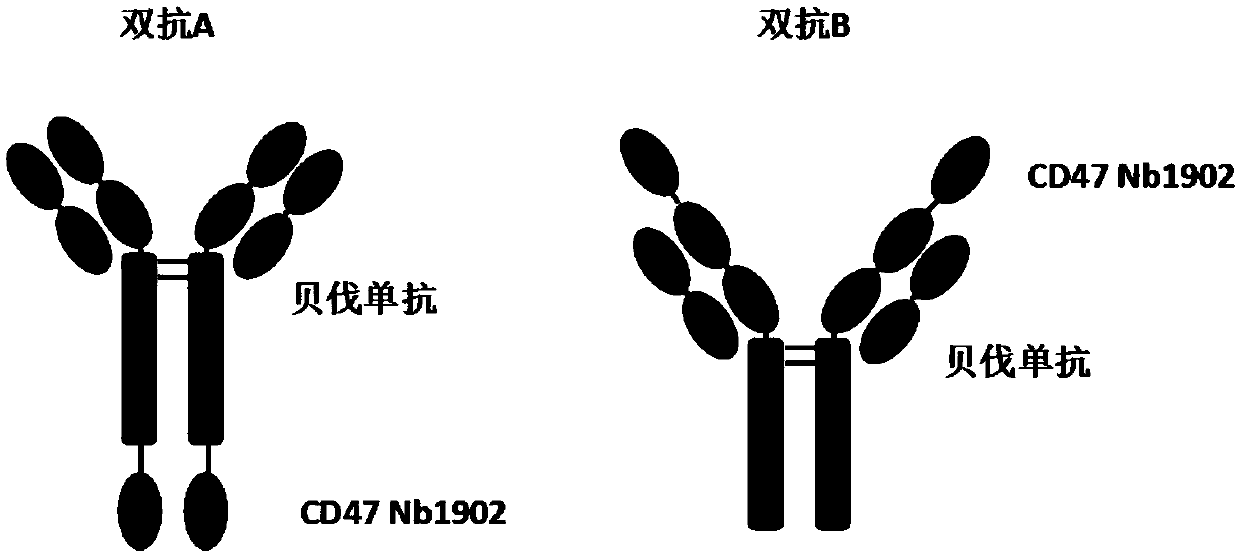
[0205] The structure of the bispecific antibody that can simultaneously bind CD47 and VEGF extracellular domain provided by the present invention is as follows: figure 1 shown. The CD47 single domain antibody sequence is derived from the patent 201810151752.6; then the above CD47 single domain antibody is fused with the heavy chain of the Bevacizumab monoclonal antibody, and the heavy chain is fused by Linker ((GGGGS)4) to the Bevacizia antibody heavy chain (LALA) and CD47 monoclonal antibody Domain antibody connection, the amino acid sequence is as follows (the underlined part is the Linker sequence):
[0206] A: The amino acid sequence is shown in SEQ ID NO.: 13, and the CD47 single domain antibody is connected to the C-terminal of the heavy chain of the bevacizumab through a linker (GGGGS) 4;
[0207] EVQLVESGGGLVQPGGSLRLSCAASGYTFTNYGMNWVRQAPGKGLEWVGWINTYTGEPTYAADFKRRFTFSLDTSKSTAYLQMNSLRAED...
Embodiment 2
[0212] Example 2 Expression and purification of anti-CD47 / VEGF bispecific antibody
[0213]Transiently transfer the two plasmids containing the synthetic gene into HEK293F cells. The specific method is as follows: (1) Use the OMEGA plasmid answering kit to prepare a large number of plasmids containing the heavy chain and the plasmid containing the light chain respectively. Filter and sterilize in the stage for standby; (2) Cultivate HEK293F cells to 2.0×10 6 each / mL; (3) Mix the heavy chain plasmid and the light chain plasmid according to the mass ratio of 2:3, and mix the mixed plasmid with the transfection reagent PEI at a ratio of 1:3 to the transfection medium F17 (Gibco) Stand in the middle for 20min, then add to HEK293F cells, at 37℃, 6%CO 2 Cultivate in a shaker incubator for 6 days; (4) Centrifuge to obtain the supernatant, combine the supernatant with protein A beads at room temperature for 1 hour; (5) Use pH 7.0 phosphate buffer to wash away foreign proteins and oth...
Embodiment 3
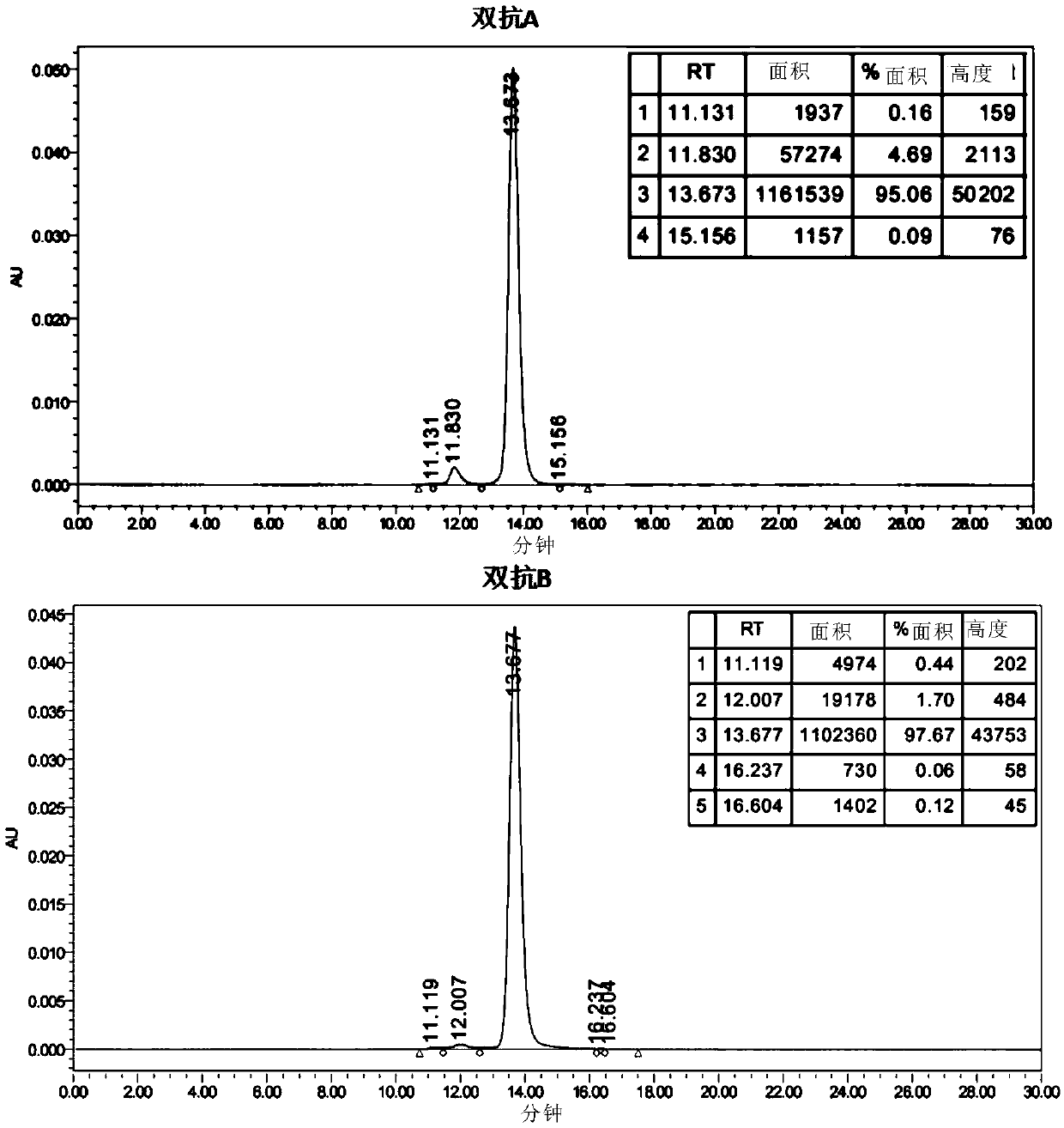
[0216] Example 3 Candidate Double Antibody A Purity Analysis
[0217] The purified sample bis-antibody A was subjected to SEC analysis: (1) Sample preparation: Dilute the sample to 1.0 mg / mL with mobile phase as the test sample. Use a 0.22um syringe filter to filter into a sample vial and put it into an HPLC autosampler for sample injection. (2) Mobile phase: 200mM phosphate aqueous solution with pH 7.0, chromatographic column: X bridge BEH200SEC 3.5μm 7.8x300mm, the parameters are set as follows: detection wavelength 280nm, column temperature 25℃, flow rate 0.5mL / min, injection volume 10uL. (3) Test results such as figure 2 As shown, the purity of double antibody A reached 95.06%, and the purity of double antibody B reached 97.67%, which can be used in subsequent experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com