Recombinant virus vector, immune composition containing same, and use
A technology of recombining viral vectors and antigens, applied in the fields of molecular biology and immunology, can solve problems such as not achieving the desired effect, and achieve the effect of prolonging survival time and inhibiting the growth of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
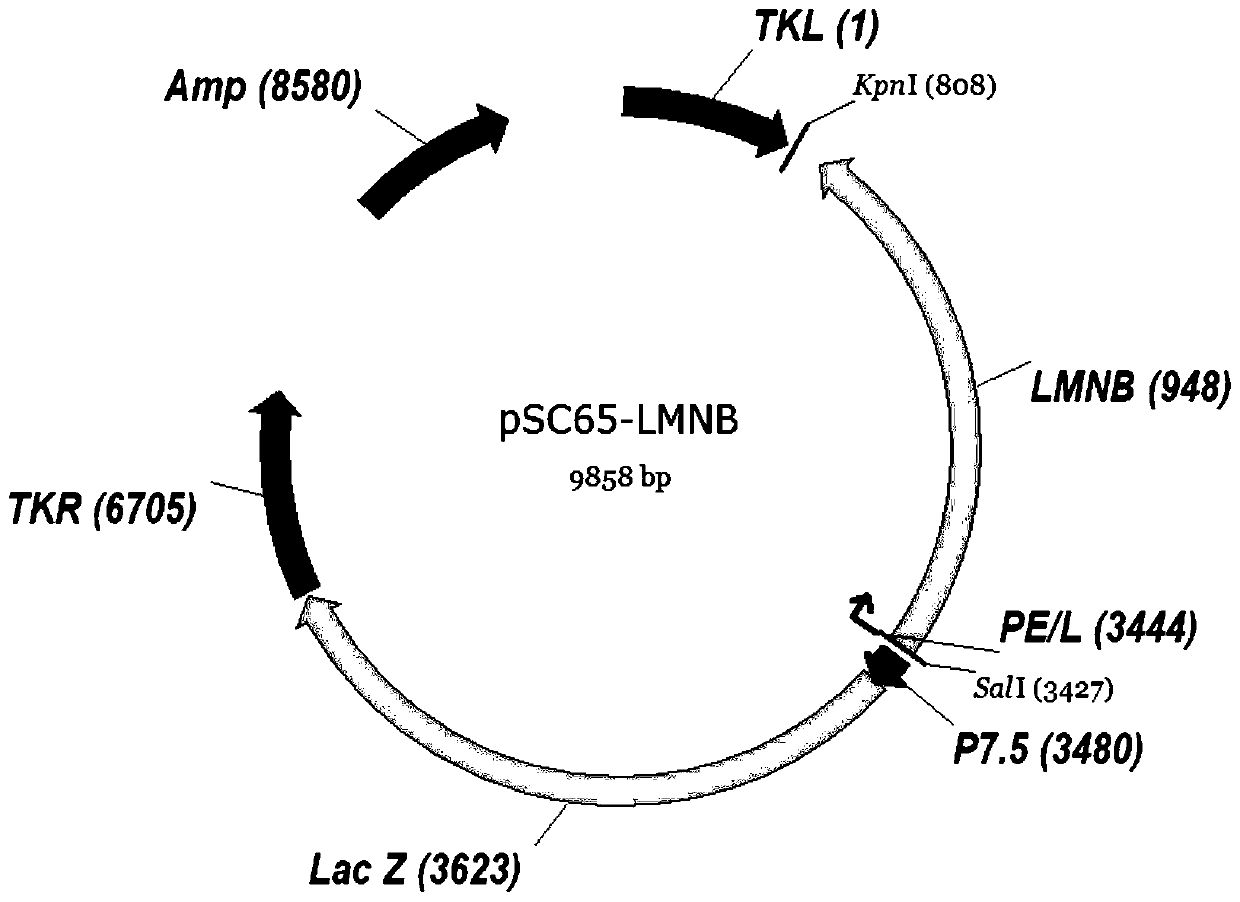
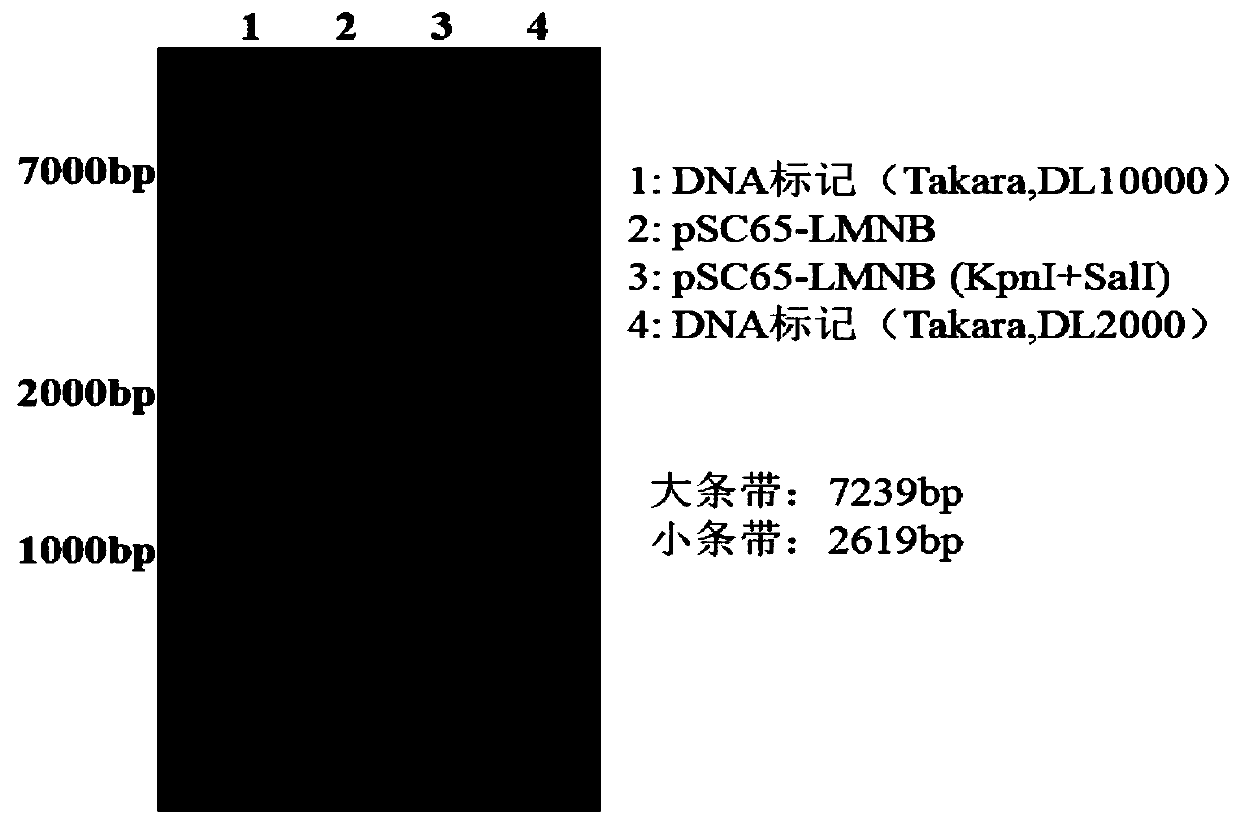
[0041] Example 1 Shuttle vector pSC65-LMNB construction
[0042] The eukaryotic expression vector pVKD1.0-LMNB expressing the triple fusion tumor antigens LAGE-1, MAGE-A3, NY-ESO-1 and cholera toxin B subunit was provided by Suzhou Industrial Park Weida Biotechnology Co., Ltd. (refer to CN109575142A) , wherein, the amino acid sequence of the LAGE-1 antigen is shown in SEQ ID NO: 1, its encoding nucleic acid sequence is shown in SEQ ID NO: 2; the amino acid sequence of the MAGE-A3 antigen is shown in SEQ ID NO: 3 Its coding nucleic acid sequence is shown in SEQ ID NO: 4; the amino acid sequence of the NY-ESO-1 antigen is shown in SEQ ID NO: 5, and its coding nucleic acid sequence is shown in SEQ ID NO: 6; the described The amino acid sequence of the cholera toxin B subunit polypeptide is shown in SEQ ID NO: 7, and its coding nucleic acid sequence is shown in SEQ ID NO: 8; the amino acid of LMNB fusion protein is shown in SEQ ID NO: 9, and its nucleic acid coding sequence is s...
Embodiment 2
[0045] Example 2 Construction of recombinant vaccinia virus vector rvv-LMNB
[0046] The recombinant vaccinia virus vector was obtained in 143B cells, and the specific method was as follows. On the first day, 143B cells were plated in 6-well cell culture plates (JET, TCP-010-006) ( CRL-8303), 1×10 6 / well and incubated overnight in a 37°C carbon dioxide cell incubator. On the second day, at 0.05 MOI (i.e. 5×10 4 PFU (plaque forming unit) / well) was added to vaccinia virus wild strain 752-1 (provided by Beijing Biological Products), and then placed in a 37°C carbon dioxide cell incubator and incubated for two hours, during which the shuttle vector / transfection reagent was prepared Complex. The shuttle vector is pSC65-LMNB obtained in Example 1, and the transfection reagent is Turbofect (Thermo Fisher Scientific, R0531). For the transfection dose and compounding method, please refer to the instructions of the transfection reagent. After the complex system was completed, t...
Embodiment 3
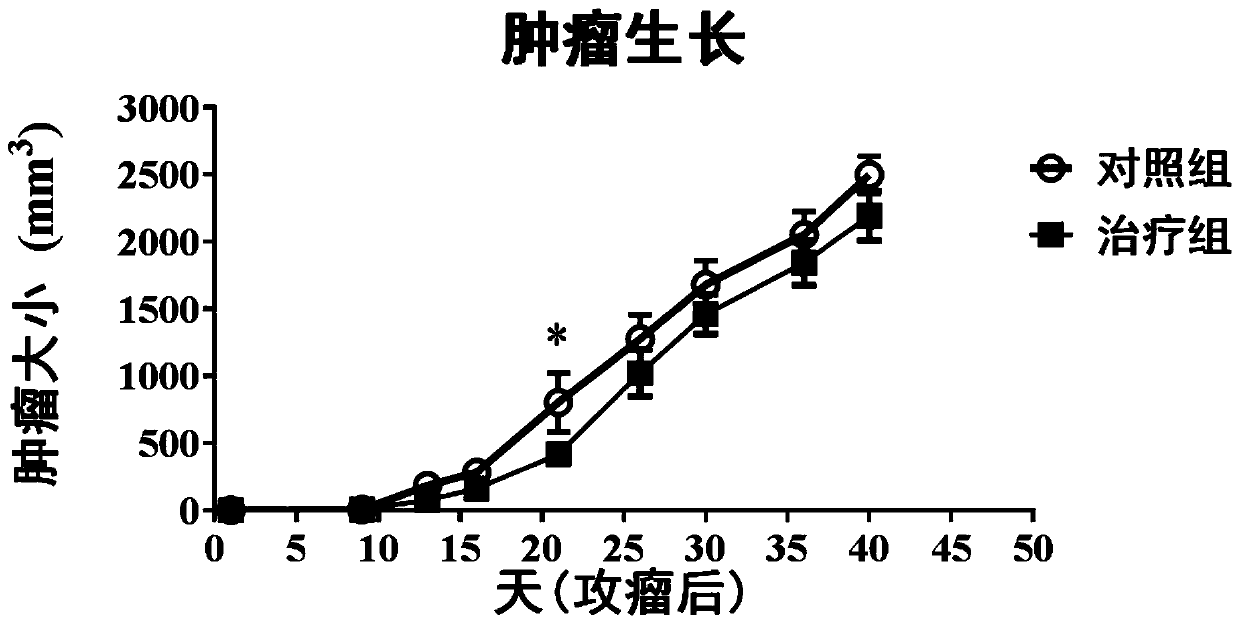
[0055] Example 3 Amplification preparation and titration of recombinant vaccinia virus vector rvv-LMNB
[0056] The recombinant vaccinia virus vector rvv-LMNB constructed in Example 2, and the wild strain of vaccinia virus were respectively grown in Vero cells ( CCL-81) was amplified, and the amplification method was as follows.
[0057] The day before, prepare a 100% confluent Vero monolayer (1×10 7 cells / dish), a total of 10 dishes.
[0058] Remove the supernatant, replace with the maintenance medium, inoculate the poxvirus to be amplified on the cells (0.01PFU / cell), incubate in a 37°C incubator for 2-3 days, and observe obvious cytopathic changes.
[0059] The cells were scraped and collected, centrifuged at 1800 g for 5 minutes, and the supernatant was removed.
[0060] Resuspend with 5 mL of maintenance medium, and sonicate on ice with an ultrasonic cell pulverizer, the sonication conditions are: 50 watts, 5 seconds of ultrasound / 5 seconds interval, a total of 15 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com