Marker for predicting efficacy of neoadjuvant chemotherapy for breast cancer patients and application of marker
A breast cancer, patient technology, applied in the determination/examination of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve problems such as incomplete elucidation of molecular mechanisms, influence on treatment plans, and differences in efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
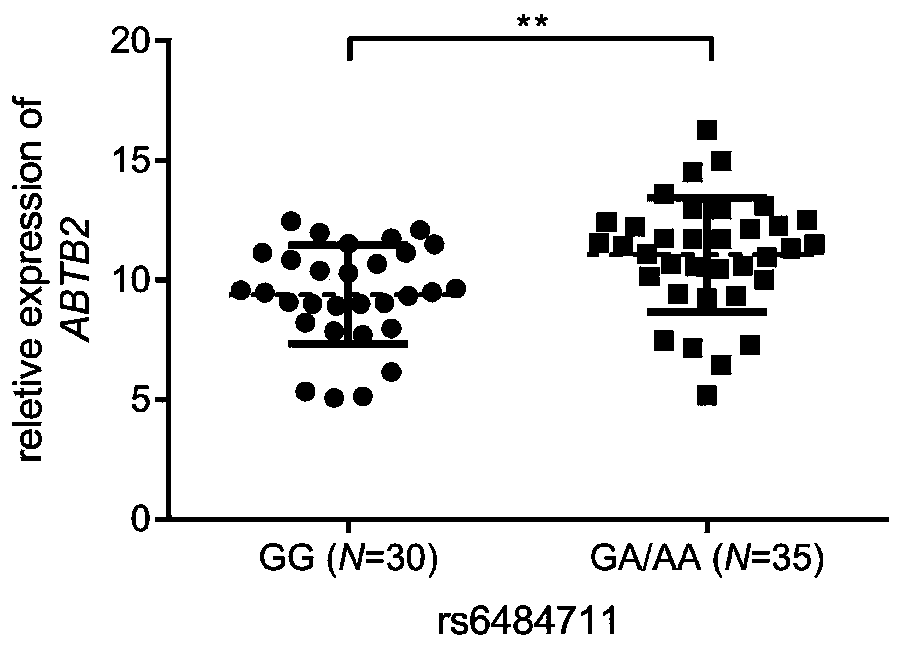
[0031] The screening of embodiment 1 candidate SNP
[0032] 1. Preliminary screening of candidate SNPs
[0033]Firstly, genes related to resistance / sensitivity to epirubicin (NCI No. 256942) or docetaxel (NCI No. 628503) were screened in the CellMiner database (http: / / discover.nci.nih.gov / cellminer / ). Using Pearson's correlation coefficient and growth inhibition value (GI50, a measure of cell line sensitivity) for gene expression below -0.4 or above 0.4 as a criterion, 284 epirubicin genes and 228 docetaxel genes were obtained. Considering redundancy, 511 genes were removed and considered as potential biomarkers of resistance or susceptibility. We then obtained all SNPs located 5 kb upstream and genes in Han with a minor allele frequency (MAF) >0.05 from the 1000 Genomes Project (http: / / www.1000genomes.org / ). Finally, the ANNOVAR software tool was applied to annotate the function of the genetic variation, and 14 SNPs were prioritized as candidate regulatory SNPs for the foll...
Embodiment 2
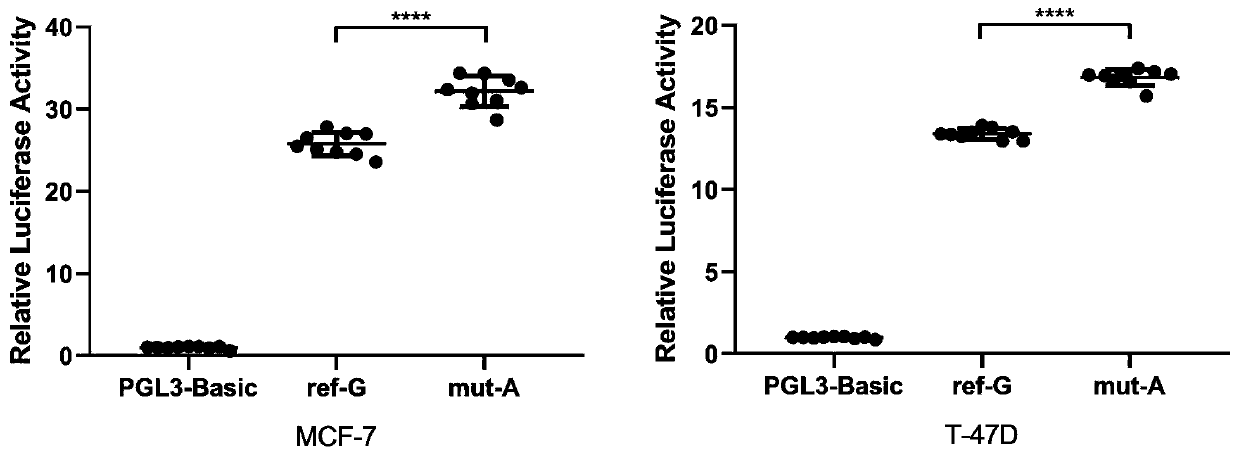
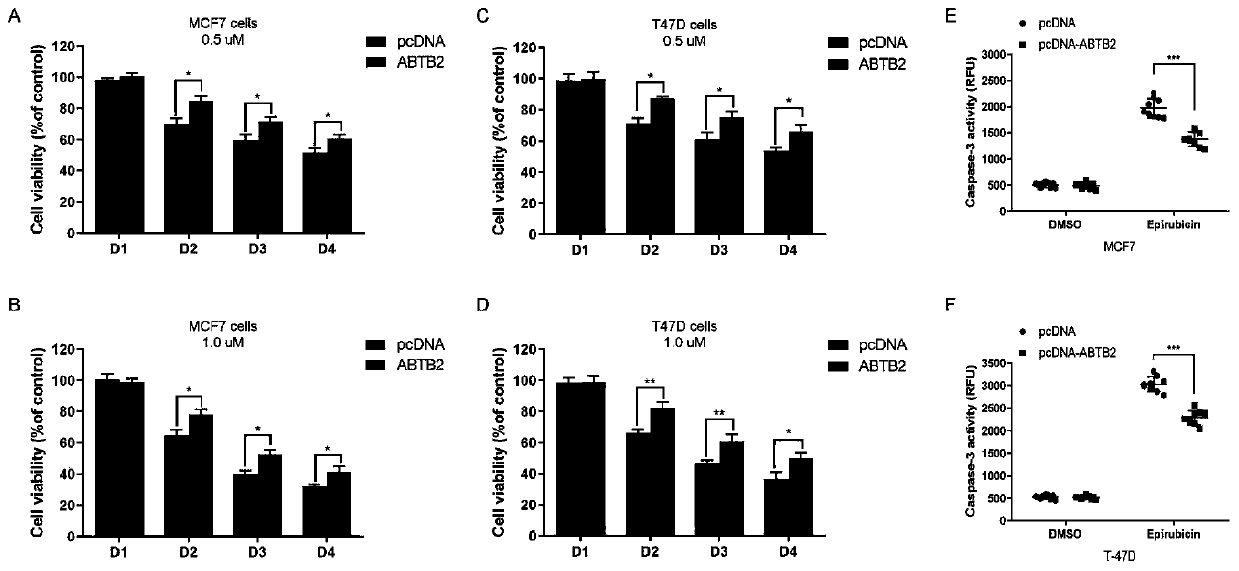
[0064] Embodiment 2, biological verification
[0065] 1. Luciferase reporter assay
[0066] Plasmid construction
[0067] DNA fragments containing rs6484711[G] or rs6484711[A] were subcloned into pGL3-Basic vector (Promega, USA), respectively. The full-length cDNA of ABTB2 was subcloned into pcDNA3.1(+) vector (Invitrogen, USA). All recombinant plasmids were synthesized and sequence verified by Genewiz (Suzhou, China).
[0068] cell culture
[0069]Human MCF-7 and T-47D BC cell lines were purchased from China Type Culture Collection (Wuhan, China). Cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) or Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 1% antibiotics (100 U / mL penicillin and 0.1 mg / mL streptomycin) at 37°C in a humidified environment of 5% CO2. All cell lines were routinely tested by DNA sequencing using the Applied Biosystems AmpF / STR Identifier kit and teste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com