Method for improving stability of low-concentration atropine ophthalmic preparation
A stable, atropine sulfate technology, applied to atropine ophthalmic preparations, the field of preparing said ophthalmic preparations, can solve problems such as increased preparation irritation, product safety impact, restricted product development and promotion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
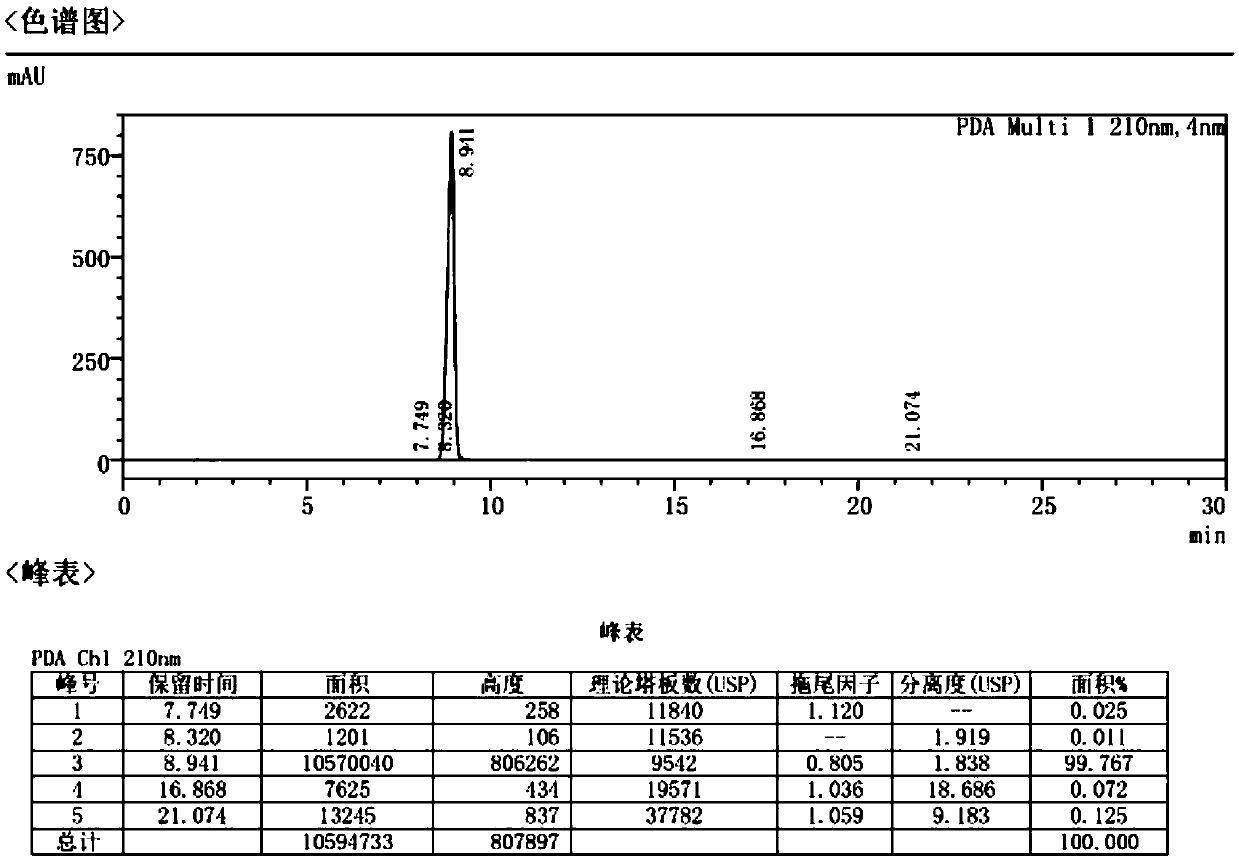
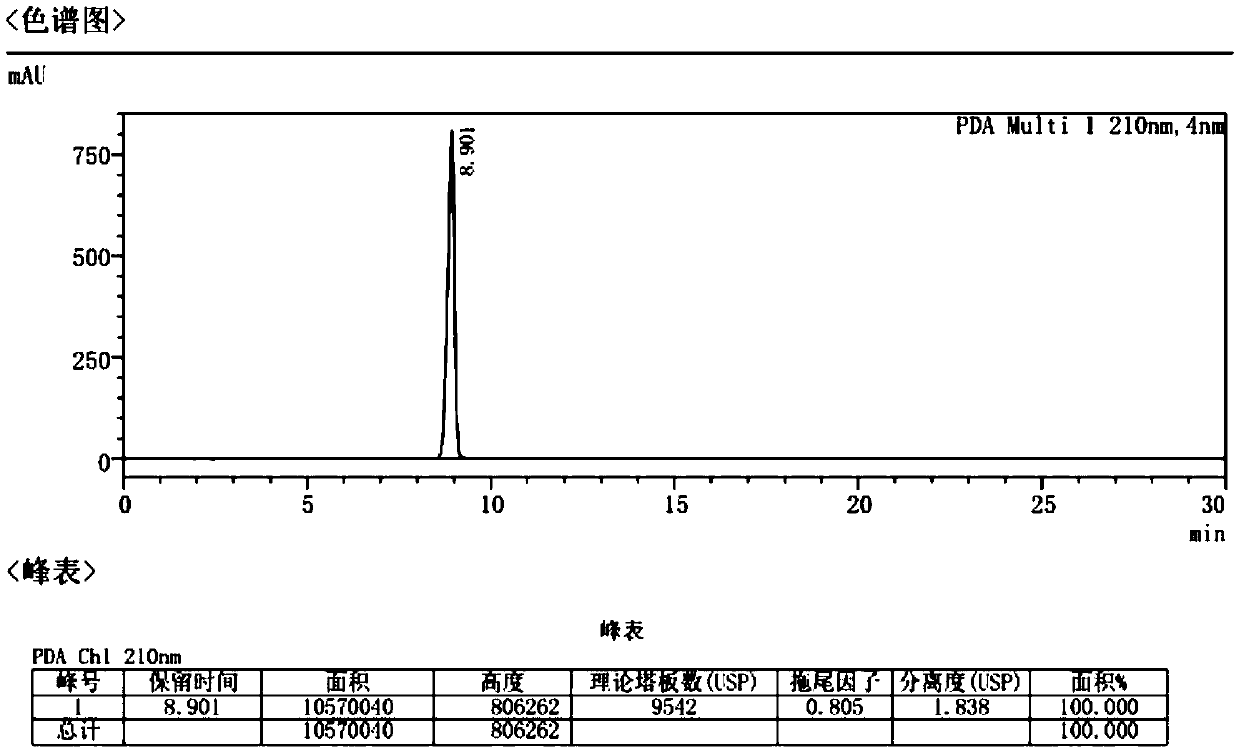
[0059] Example 1 Refining of Atropine Sulfate API
[0060] Place the commercially available atropine sulfate crude drug A or B with a purity of more than 99% in a pulverizer with a 60-mesh sieve, slowly pulverize and sieve, and collect the raw materials under the sieve for subsequent use. Take 50 g of pulverized raw materials and place them in a 3000 mL three-neck flask, add 20 times the amount of anhydrous acetone, stir and wash at 40°C for 3 hours, and filter with suction to obtain wet product 1. Take the wet product 1, put it in a 3000mL three-neck flask, add 15 times the amount of 5% acetone aqueous solution, stir and wash at 40°C for 4 hours, and filter with suction to obtain the wet product 2. The wet product 2 was placed in a 1000mL three-neck flask, 10 times the amount of acetone was added, stirred and washed at 5°C for 1.5h, suction filtered, and dried under reduced pressure for 6h to obtain 41g of atropine sulfate, yield: 82%. HPLC carries out impurity analysis, t...
Embodiment 2~ Embodiment 6
[0071] According to the corresponding prescription ratio in Table 3, the atropine ophthalmic preparation was prepared by the following preparation method.
[0072] Preparation:
[0073] (1) Take 10g of water for injection at 80-90°C, add the prescribed amount of hydroxypropyl methylcellulose or sodium hyaluronate to fully disperse and swell, make up to 20g of water for injection below 30°C, stir and dissolve to a transparent solution, and set aside;
[0074] (2) Take 50g of water for injection at 65-75°C, dissolve the prescribed amount of sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate, disodium edetate and benzalkonium chloride in turn, put it below 30°C, and add the prescribed amount The atropine sulfate refined by the method in Example 1, stirring and dissolving;
[0075] (3) mix the hydroxypropyl methylcellulose solution of (1) gained with the solution of (2) gained;
[0076] (4) Add water to the mixed solution obtained in (3) to the full amount of 1...
Embodiment 7~ Embodiment 9
[0081] According to the corresponding prescription ratio in Table 4, the atropine ophthalmic preparation was prepared by the following preparation method.
[0082] Preparation:
[0083] (1) Take 10g of water for injection at 80-90°C, add the prescribed amount of hydroxypropyl methylcellulose to fully disperse and swell, add water for injection below 30°C to 20g, stir and dissolve to a transparent solution, and set aside;
[0084] (2) Take 50g of water for injection at 65-75°C, dissolve the prescribed amount of boric acid, edetate disodium and benzalkonium chloride in turn, put it below 30°C, add the prescribed amount of refined according to the method in Example 1 Atropine sulfate, stirred to dissolve;
[0085] (3) mix the hydroxypropyl methylcellulose solution of (1) gained with the solution of (2) gained;
[0086] (4) Add water to the mixed solution obtained in (3) to the full amount of 100g, stir evenly, filter and sterilize with a 0.22 μm filter membrane, and fill.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com