Method for selective recovery of valuable metal from waste denitrification catalyst through alkali fusion
A technology for denitrification catalyst and valuable metals, applied in the direction of improving process efficiency, etc., can solve the problems of separation and recovery of valuable metals, increased roasting time, and no separation and recovery of titanium dioxide.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
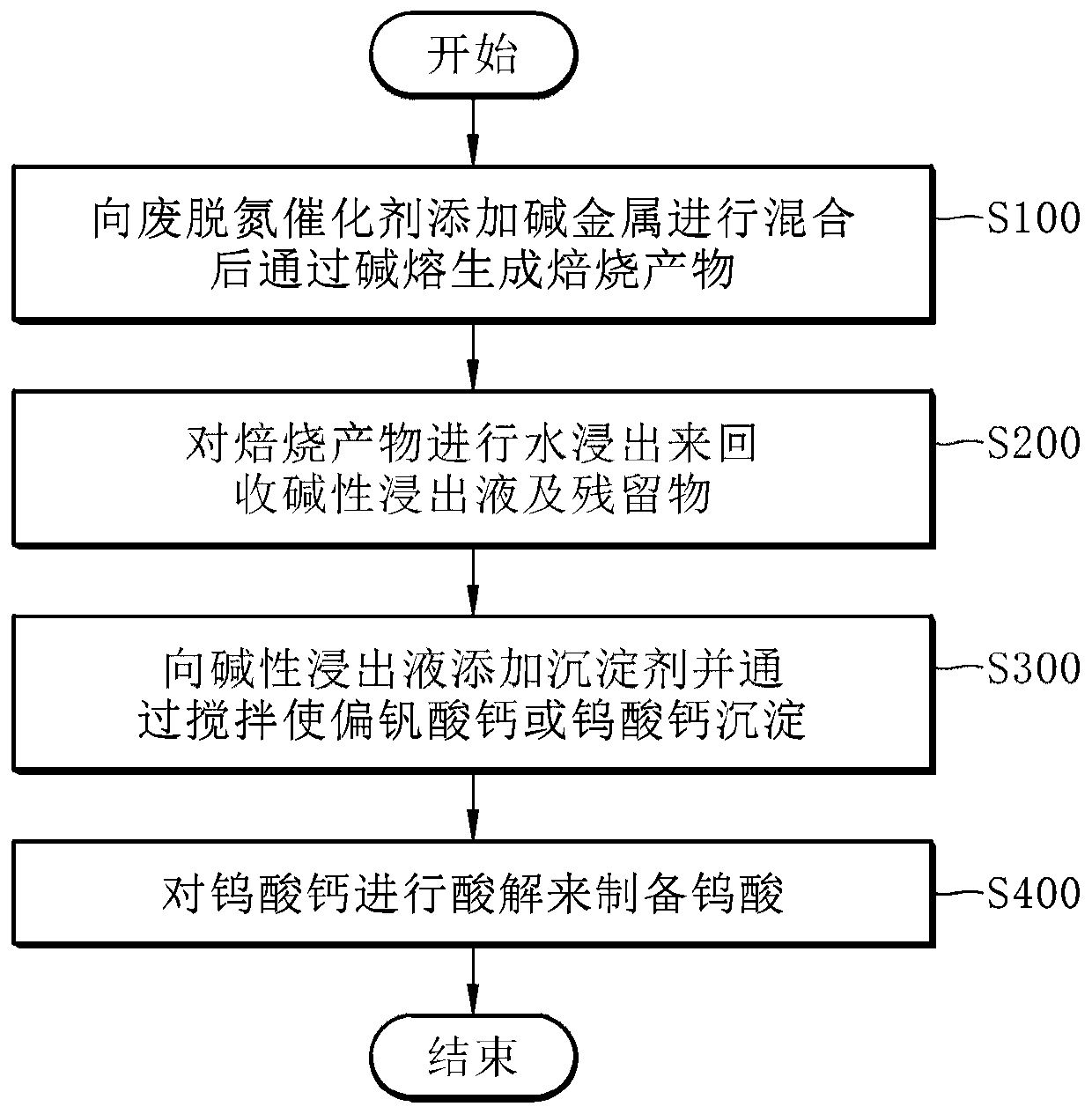
[0059] figure 1 A process flow of a method for selectively recovering valuable metals from spent denitrification catalysts by alkali melting according to an embodiment of the present invention is shown.
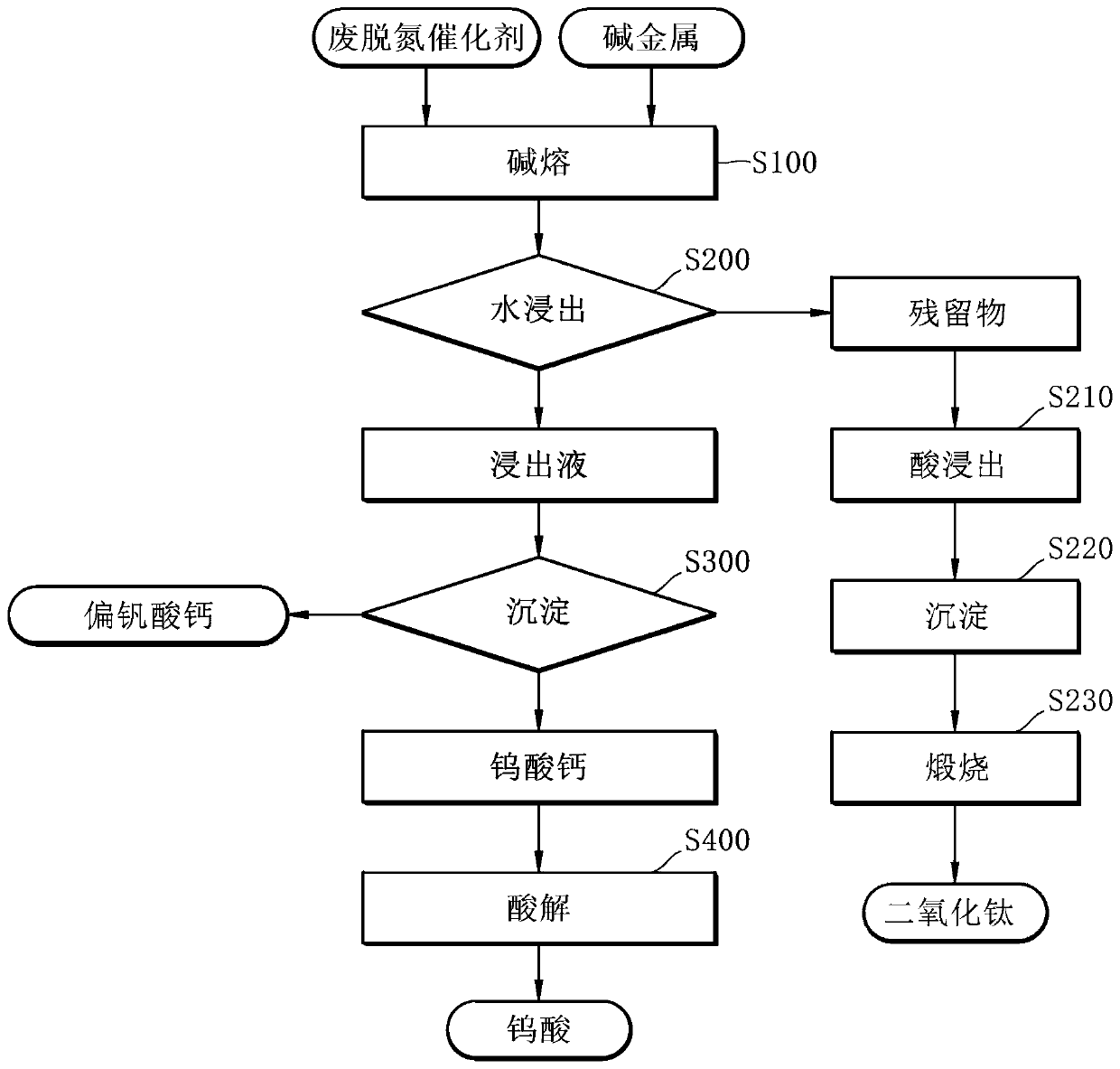
[0060] refer to figure 1 , the method for selectively recovering valuable metals from spent denitrification catalysts by alkali fusion comprises: step (a), adding alkali metals to waste denitrification catalysts for mixing and generating roasted products by alkali fusion; step (b) , by leaching the above-mentioned roasted product with water to recover the alkaline leach solution and the residue; step (c), adding a precipitant to the above-mentioned alkaline leach solution, and recovering by stirring to precipitate calcium metavanadate or calcium tungstate; and In step (d), the recovered calcium tungstate is subjected to acid hydrolysis to prepare tungstic acid.
[0061] Specific Embodiments of the Invention
[0062] Hereinafter, preferred embodiments of the present inventi...
Embodiment 1
[0137] Embodiment 1. selectively recovers valuable metals by alkali fusion
[0138] The spent denitrification catalyst was prepared and pulverized, and analyzed by inductively coupled atomic emission spectrometry (ICP-AES).
[0139] It is confirmed that it is an oxide form of vanadium, tungsten, and titanium, and the firing conditions for alkali fusion are derived.
[0140] First prepare the spent denitrification catalyst of 300g, add 1.2 equivalents of sodium hydroxide (NaOH) relative to the spent denitrification catalyst, and in order to reclaim titanium, add 2 equivalents of sodium hydroxide relative to the spent denitrification catalyst, with the same Method for alkali fusion.
[0141] The power of microwave is adjusted to 3kw, alkali fusion is carried out for 20 minutes at 1.2 equivalents, and alkali fusion is carried out at 2 equivalents for 60 minutes.
[0142] Alkali fusion is carried out at a temperature of 900°C to 1000°C.
[0143] After alkali fusion, the pulp de...
Embodiment 2
[0146] Embodiment 2. selectively recover titanium dioxide
[0147] Hydrochloric acid was added to the residue of Example 1 to adjust the pH to 2 or less, and the reaction was carried out at a temperature of 50° C. for 2 hours to perform acid leaching. Sodium hydroxide was added again for precipitation, and the recovered precipitate was calcined at a temperature of 900K for 5 hours to recover titanium dioxide with a purity of 90% or more.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com