Spiropyrane for improving solid photochromic performance by introducing rigid group and synthesis method thereof
A photochromic and synthetic method technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, etc., can solve the problem of high commercialization cost and inability to prepare photochromic polymer materials , Organic photochromic materials change color speed and small free volume of molecules, etc., to achieve the effect of improving color changing performance and fatigue resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
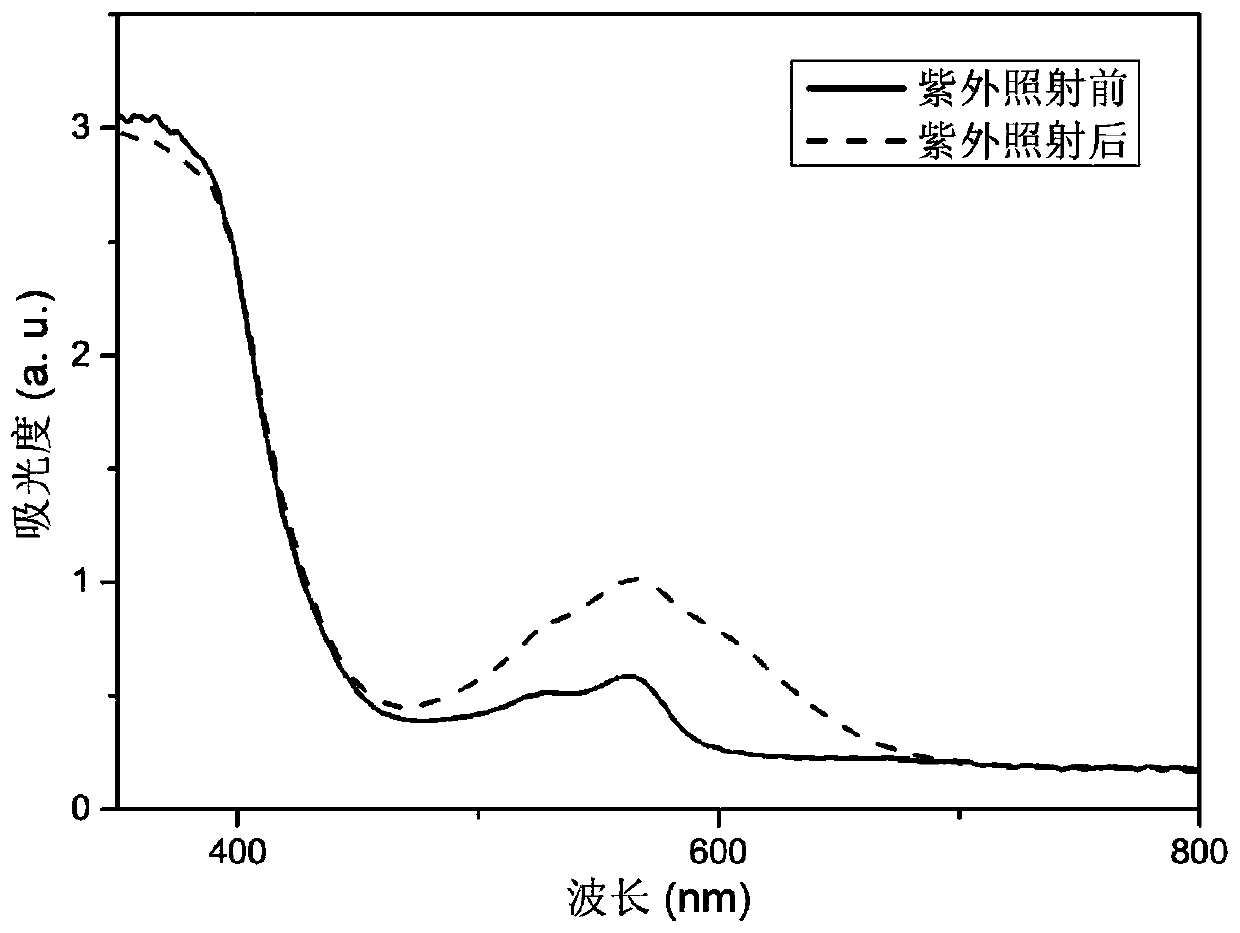
Image
Examples
Embodiment 1
[0022] In this embodiment, a spiropyran that introduces a rigid group to improve the solid-state photochromic performance, the nitrogen atom of the spiropyran in the indole ring is connected to the ethanol group, and the rigid aromatic ring molecule is introduced into the ethanol group by reacting with the hydroxyl group of the ethanol group. Among spiropyrans; spiropyrans introduced into rigid groups through modification have the following structure:
[0023]
[0024] The synthetic reaction formula is as follows:
[0025]
[0026] A kind of synthetic method that introduces the spiropyran of rigid group of the present embodiment, described method comprises the steps:
[0027] To 10 ml of ultra-dry dichloromethane was added 3,5-bis(benzyloxy)benzoic acid (3.42 mmol, 1.14 g), 1-ethyl-3(3-dimethylpropylamine)carbodiimide (EDCI) (3.42mmol, 0.66g) and 4-dimethylaminopyridine (DMAP) (3.42mmol, 0.42g), stirred under nitrogen until a clear solution. Dissolve 1'-(2-hydroxyethyl...
Embodiment 2
[0031] In this embodiment, a spiropyran that introduces a rigid group to improve the solid-state photochromic performance, the nitrogen atom of the spiropyran in the indole ring is connected to the ethanol group, and the rigid aromatic ring molecule is introduced into the ethanol group by reacting with the hydroxyl group of the ethanol group. Among spiropyrans; spiropyrans introduced into rigid groups through modification have the following structure:
[0032]
[0033] The synthetic reaction formula is as follows:
[0034]
[0035] To 10 ml of ultra-dry dichloromethane was added 3,5-bis(benzyloxy)pentafluorobenzoic acid (0.89 mmol, 0.47 g), 1-ethyl-3(3-dimethylpropylamine)carbodiimide ( EDCI) (2.68mmol, 0.52g) and 4-dimethylaminopyridine (DMAP) (2.68mmol, 0.32g), stirred under nitrogen atmosphere until clear solution. SP (0.89mmol, 0.31g) was dissolved in 10ml of anhydrous dichloromethane, added dropwise to the above clear solution, and reacted at room temperature for 2...
Embodiment 3
[0039] In this embodiment, a spiropyran that introduces a rigid group to improve the solid-state photochromic performance, the nitrogen atom of the spiropyran in the indole ring is connected to the ethanol group, and the rigid aromatic ring molecule is introduced into the ethanol group by reacting with the hydroxyl group of the ethanol group. Among spiropyrans; spiropyrans introduced into rigid groups through modification have the following structure:
[0040]
[0041] The synthetic reaction formula is as follows:
[0042]
[0043] To 10 ml of ultra-dry dichloromethane was added pentafluorobenzoic acid (6.55 mmol, 1.38 g), EDCI (1-ethyl-3(3-dimethylpropylamine) carbodiimide) (9.82 mmol, 1.88 g) and DMAP (4-dimethylaminopyridine) (9.82 mmol, 1.20 g), stirred under nitrogen atmosphere until clear solution. SP (1.31mmol, 0.46g) was dissolved in 10ml of anhydrous dichloromethane, added dropwise to the above clear solution, and reacted at room temperature for 24 hours. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com