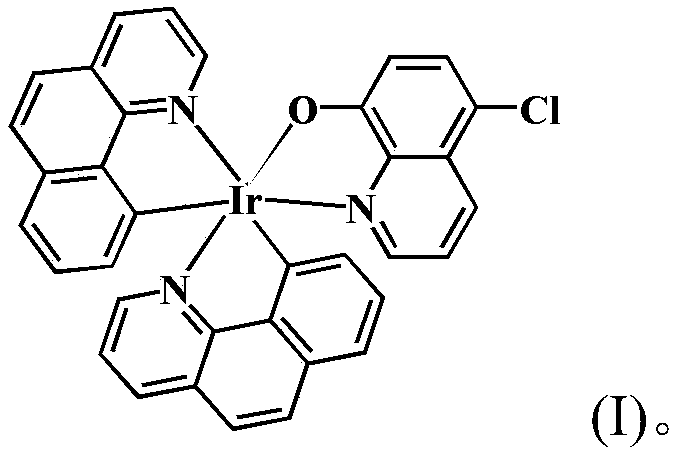
Iridium complex as well as synthesis method and application thereof
A synthetic method and compound technology, applied in the field of medicine, can solve the problem of low liver cell toxicity and achieve significant biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 100.0mL round bottom flask, add 2.0mmol of 5-chloro-8-hydroxyquinoline and 1.0mmol of 7,8-benzoquinoline iridium dimer, and then add 15.5mL of organic solvent (from 15.0mL of ethanol and 0.5mL of dimethyl sulfoxide), stirred to dissolve, reacted at 55°C until complete (about 8h), stopped the reaction, cooled to room temperature, reddish-brown crystals were precipitated, collected and dried to obtain reddish-brown solid product. Yield 79.99%.
[0021] The product obtained in this embodiment is characterized:
[0022] (1) X-ray single crystal diffraction
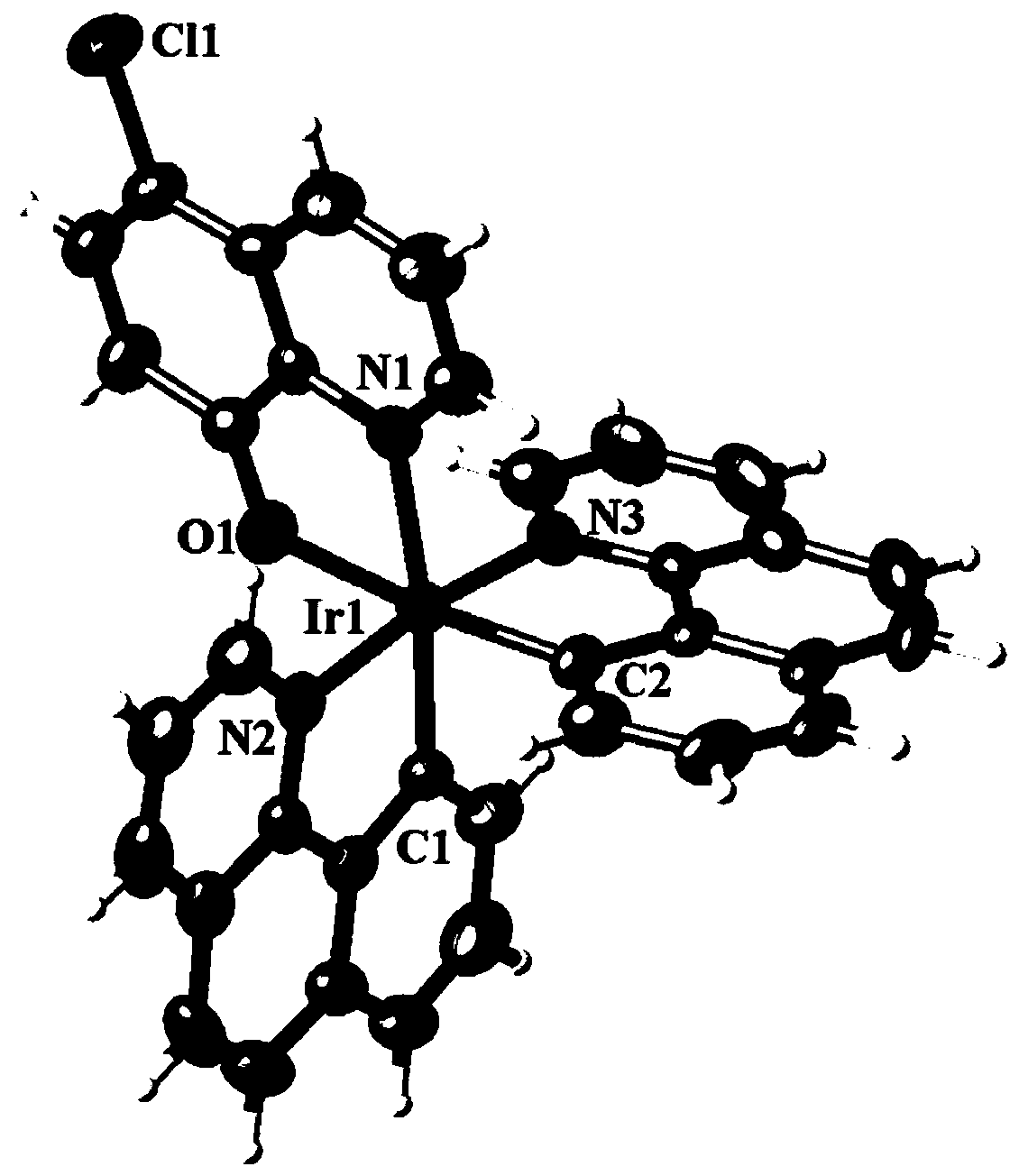
[0023] The reddish-brown crystal with perfect surface structure was measured by single crystal diffraction to determine its crystal structure. The obtained crystallographic and structural correction data are shown in Table 1 below, and the data of some bond lengths and angles are shown in Table 2 and Table 3 below. The crystal structure of reddish-brown crystals is as figure 1 shown.
[0024] Table 1. Crystall...
Embodiment 2
[0042] Example 1 was repeated, except that the organic solvent was changed to consist of 1.0 mL of ethanol and 15 mL of dichloromethane, and the reaction was carried out at 35° C. (about 50 h to complete the reaction).
[0043] The result is reddish-brown crystals. Yield 91.24%.
[0044] Single crystal diffraction analysis, elemental analysis, infrared analysis and mass spectrometry analysis were performed on the product obtained in this example, and the obtained reddish-brown crystal was determined to be the target compound.
Embodiment 3
[0046] Example 1 was repeated, except that the organic solvent was changed to consist of 3.0 mL of ethanol, 5.0 mL of water and 12.0 mL of acetone, and the reaction was carried out at 80° C. (about 35 h to complete the reaction).
[0047] As a result, reddish-brown crystals are obtained. Yield 80.55%.
[0048]Single crystal diffraction analysis, elemental analysis, infrared analysis and mass spectrometry analysis were performed on the product obtained in this example, and the obtained reddish-brown crystal was determined to be the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com