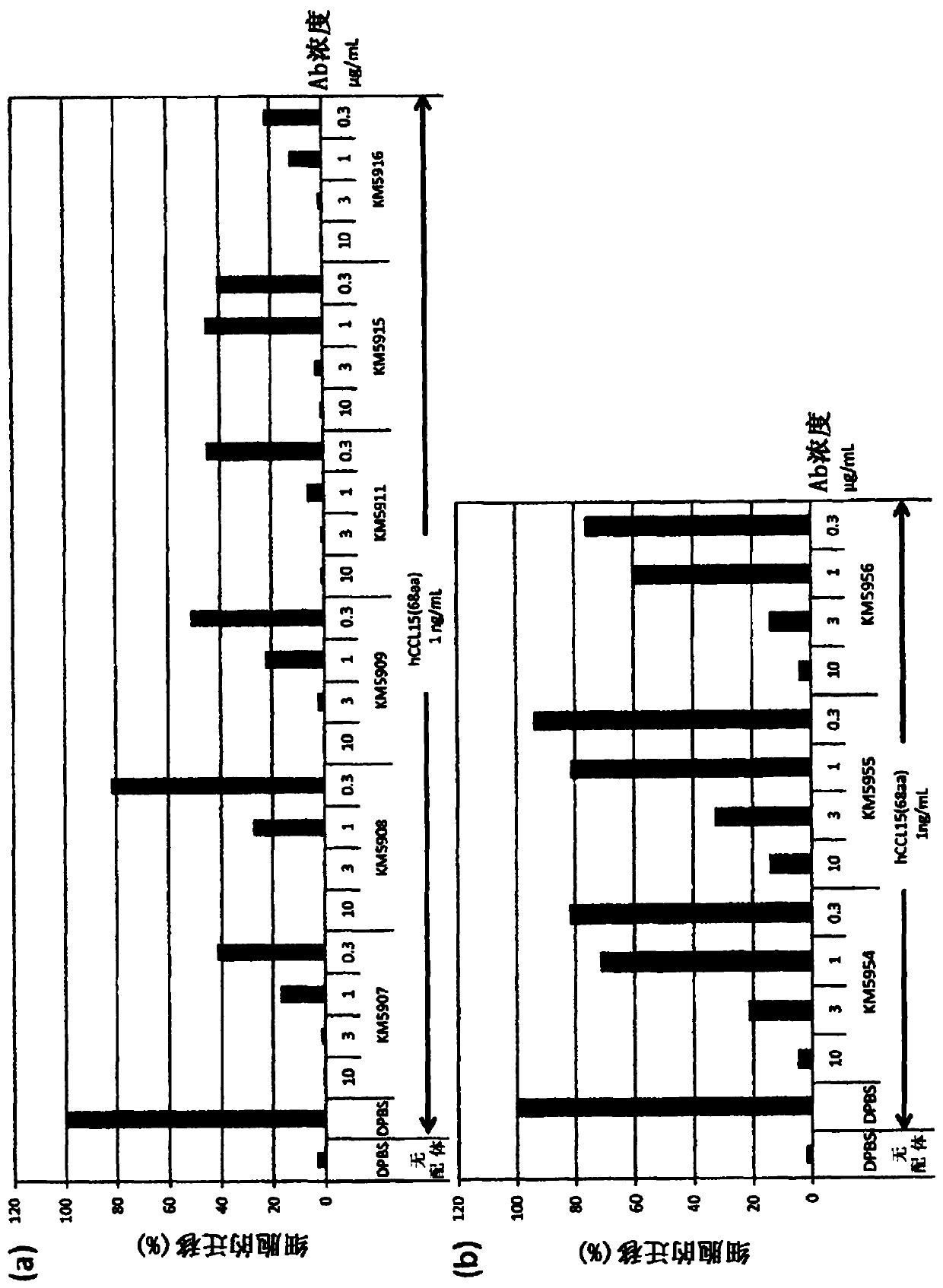
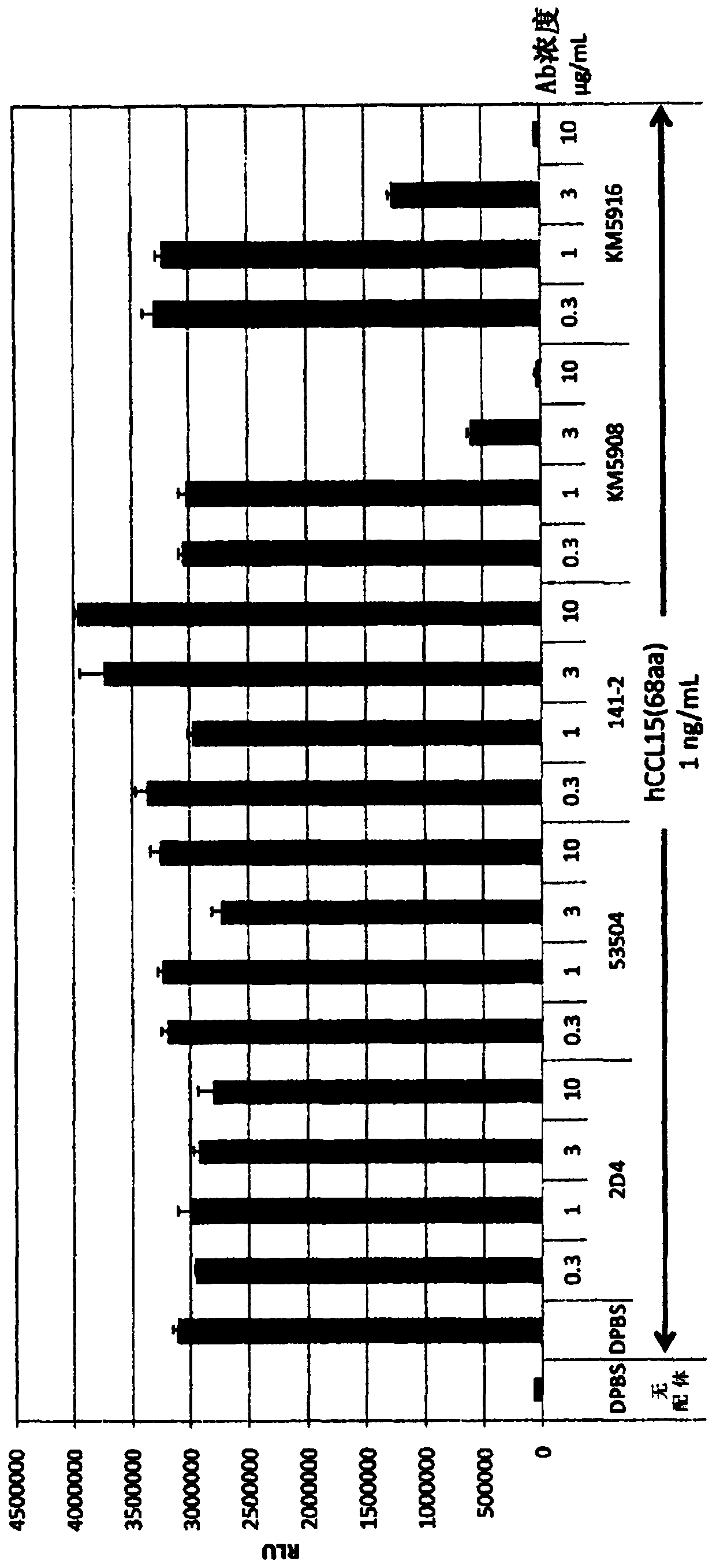
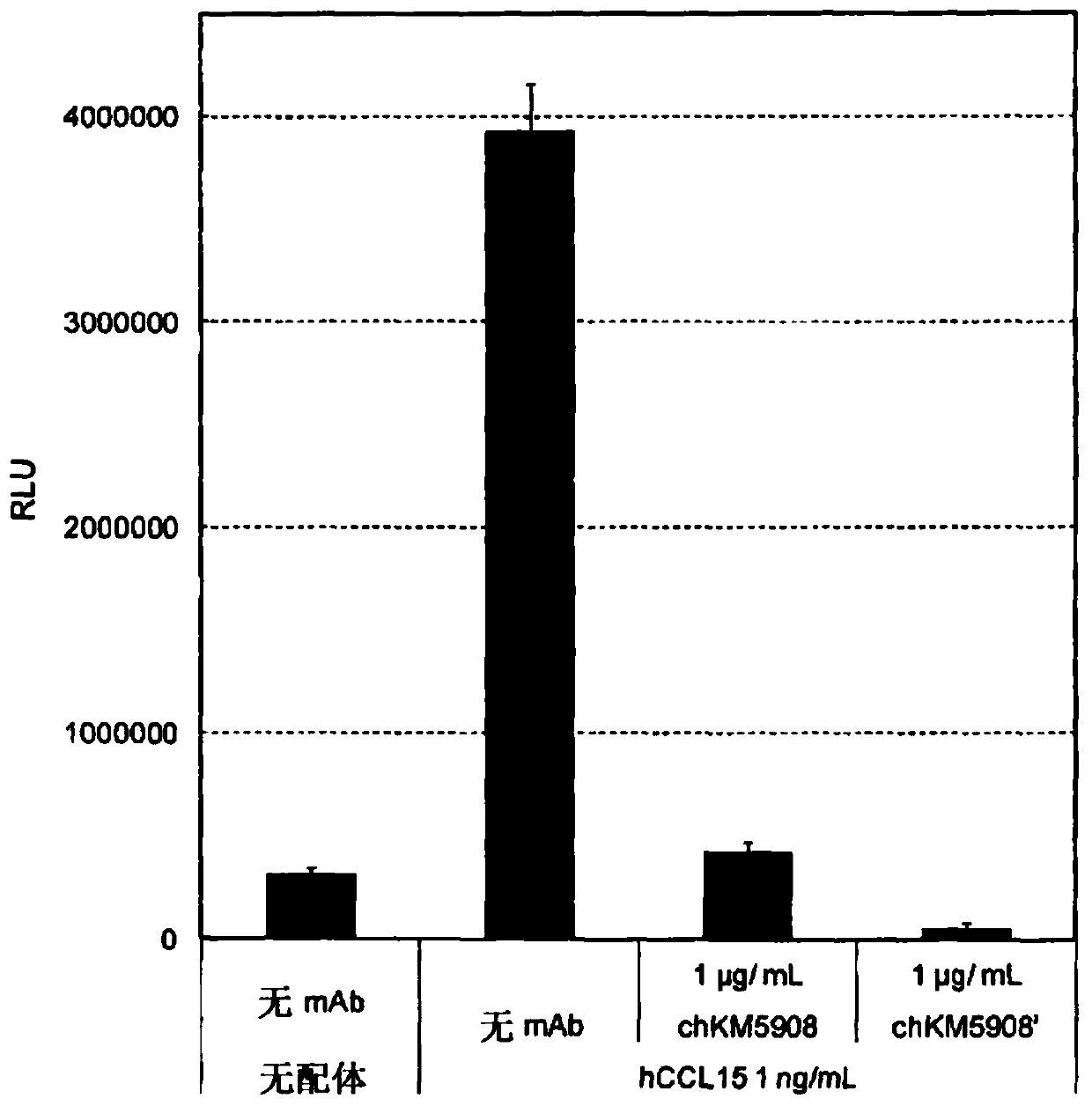
Anti-human ccr1 monoclonal antibody
A monoclonal antibody and antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-inflammatory agents, etc., can solve problems such as failure to show effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0316] (1) Antigen preparation
[0317] Human CCR1 or human CCR1-expressing cells as an antigen can be obtained by introducing an expression vector containing cDNA encoding full-length or partial-length human CCR1 into Escherichia coli, yeast, insect cells, animal cells, or the like. In addition, human CCR1 can also be obtained by purifying human CCR1 from various human cell lines, human cells, human tissues, etc. that express human CCR1 in large amounts. In addition, these human cell lines, human cells, human tissues, etc. can also be used as antigens as they are. In addition, a synthetic peptide having a partial sequence of human CCR1 can also be prepared by a chemical synthesis method such as the Fmoc method or the tBoc method, and used as an antigen. A known tag such as FLAG or His may be added to the C-terminus or N-terminus of human CCR1 or a synthetic peptide having a partial sequence of human CCR1.
[0318] Human CCR1 used in the present invention can be obtained by ...
Embodiment 1
[0462] [Example 1] Production of expression vectors for human and mouse CCR1
[0463] (1) Preparation of each CCR1 gene
[0464] DNAs encoding human or mouse CCR1 or CCR1-CCR3 chimeric receptors (GenScript Japan, Inc.) of the following 1 to 7 were synthesized. Restriction enzyme sites (BamHI and NotI) and Kozak sequences for integration into each vector were added at the time of synthesis.
[0465] 1. cDNA sequence (sequence number 1) encoding human CCR1 (hereinafter referred to as hCCR1)
[0466] 2. The cDNA sequence (sequence number 3) of coding mouse CCR1 (hereinafter referred to as mCCR1)
[0467] 3. cDNA sequence (SEQ ID NO: 5) encoding human CCR3 (hereinafter referred to as hCCR3)
[0468] 4. A cDNA sequence encoding a chimeric receptor (hereinafter referred to as NC3-hCCR1) obtained by substituting the amino acid sequence at positions 1 to 31 of human CCR1 with the corresponding N-terminal amino acid sequence of human CCR3 (SEQ ID NO: 6 )
[0469] 5...
Embodiment 2
[0478] [Example 2] Production of CCR1-expressing cell lines
[0479] (1) Production of hCCR1 expressing cells
[0480] hCCR1 / Tn-pMug-Hygro and the Tol2 transposase expression vector TPEX_pMug (International Publication No. 2013 / 005649) as the plasmid DNA prepared in Example 1 were co-introduced into CHO-S (Thermo Fisher Scientific) to prepare expressing cell lines. Gene transfer was carried out using Fugene HD (Promega) as follows. Prepare 1 × 10 inoculum in a 6-well plate 5 Cells / mL were 2.5 mL each, and a mixture of hCCR1 / Tn-pMug-Hygro, TPEX_pMug, and Fugene HD was added to the culture medium 24 hours later. 72 hours after the addition, 1 mg / mL of hygromycin (Invitrogen) was added, and drug selection was performed for about 2 weeks. The cells that had acquired drug resistance were recovered, and expression analysis was performed by flow cytometry (FACS Calibur, BD Biosciences). As a result, the expression of the introduced hCCR1 was confirmed. This cell line is r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com