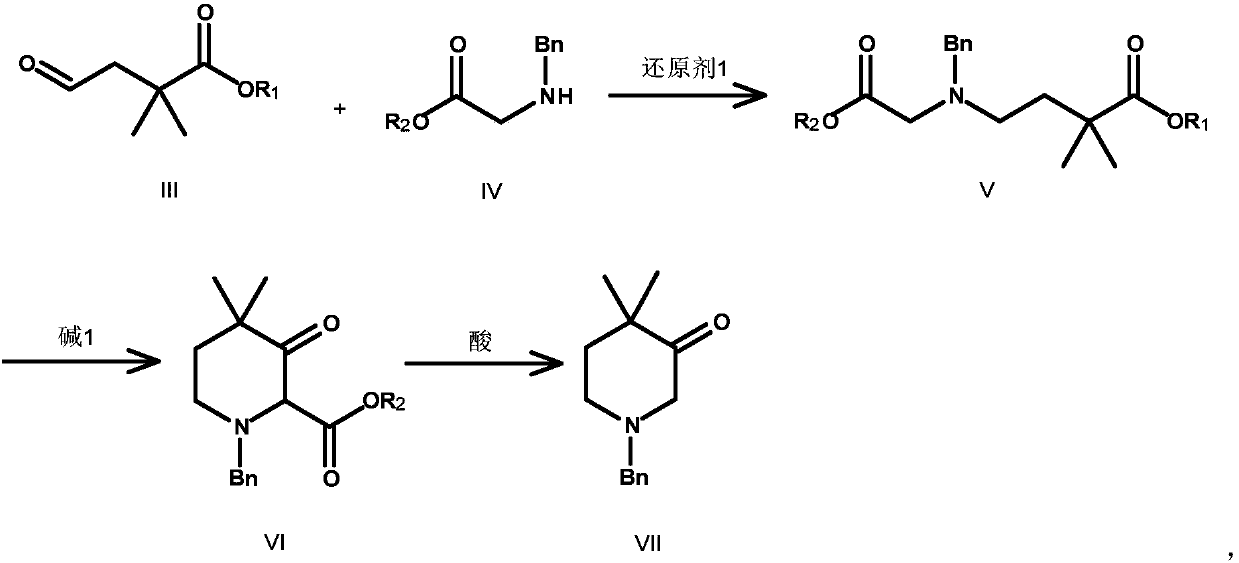
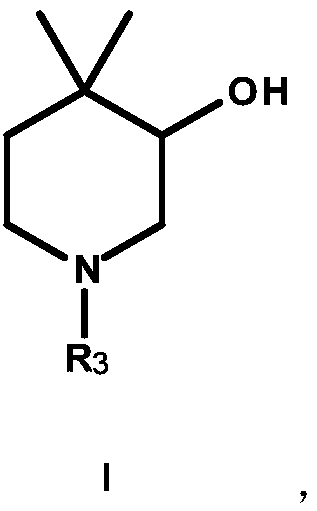
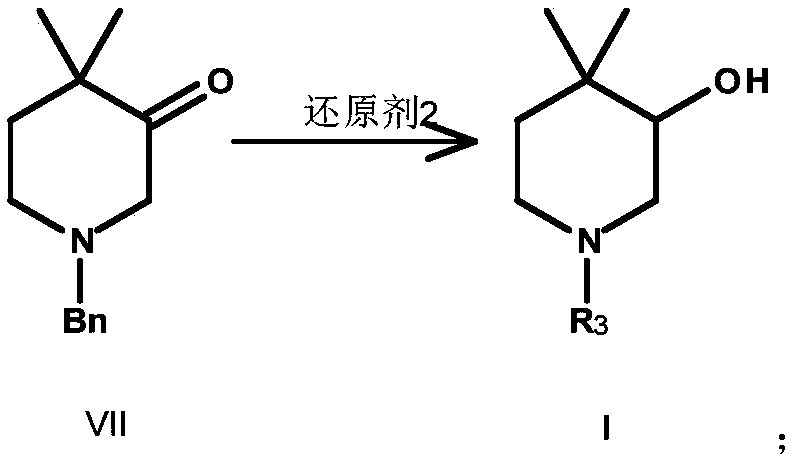
Preparation method for synthesis of N-protected and non-protected 3-hydroxy-4,4-dimethylpiperidine
A methyl and ethyl technology, applied in the field of pharmaceutical intermediate synthesis, can solve the problems such as no literature report in the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] Preparation of Compound III-1
[0047] Compound II-1 (84.18g, 0.592mol, 1.0eq.) was dissolved in 800mL ether, and H 2 O (600mL), pyridine (50.00g, 0.651mol, 1.1eq.), K 2 OSo 4 .2H 2 O (10.90g, 0.0296mol, 0.05eq.), lower the temperature to below 10°C, add sodium periodate (380.0g, 1.776mol, 3.0eq.) in batches, and stir the reaction at 0-10°C for 10h, TLC Shows complete reaction. Suction filtration, the filter cake was washed with 600mL MTBE, the combined filtrates were separated, the organic phase was washed with saturated sodium sulfite, 10% citric acid aqueous solution, sodium bicarbonate aqueous solution, saturated NaCl aqueous solution, dried and suction filtered, and the filtrate was concentrated to obtain compound III -1 is 55.00 g of black oily matter, yield 65%. 1 HNMR (400MHz, CDCl 3 ) δ (ppm): 9.75 (s, 1H), 3.71 (s, 3H), 2.67 (s, 2H), 1.31 (s, 6H).
[0048] Preparation of Compound V-1
[0049] Compound IV-1 (35.50g, 0.160mol, 1.05eq.) and co...
Embodiment 2
[0054]
[0055] Preparation of Compound III-2
[0056] Compound II-2 (92.50g, 0.592mol, 1.0eq.) was dissolved in 1000mL 1,4-dioxane, and H 2 O (600mL), 2,6-lutidine (126.87g, 1.184mol, 2.0eq.), osmium tetroxide (1.5g, 0.00592mol, 0.01eq.), lower the temperature to below 10°C, and add in batches Sodium iodate (380.0 g, 1.776 mol, 4.0 eq.) was added and stirred at room temperature for 20 h. TLC showed that the reaction was complete. Add 600mL water and 1800mL DCM, stir and separate the liquids, extract the water phase with DCM once, combine the organic phases, wash the organic phase with saturated sodium sulfite, 10% citric acid aqueous solution, sodium bicarbonate aqueous solution, saturated NaCl aqueous solution, and dry After suction filtration, the filtrate was concentrated to obtain 63.68 g of compound III-2 as a black oil, with a yield of 68%.
[0057] Preparation of Compound V-2
[0058] Compound IV-2 (28.67g, 0.160mol, 1.5eq.) and compound III-2 (16.87g, 0.106mol, ...
Embodiment 3
[0063]
[0064] Preparation of Compound III-3
[0065] Compound II-3 (20.01g, 0.117mol, 1.0eq.) was dissolved in 200mL DCM / MeOH mixed solvent, cooled to below -78°C, and passed O 3 Gas for 30 min, PPh3 (36.7 g, 0.14 mol, 1.2 eq.) was added at -78 ° C, and the reaction was stirred at room temperature for 3 h after the addition was completed. TLC showed that the reaction was complete. The reaction solution was concentrated and distilled under reduced pressure to obtain 14.1 g of compound III-3 as a yellow oil, with a yield of 70%.
[0066] Preparation of Compound V-3
[0067] Compound IV-3 (28.17g, 0.146mol, 1.8eq.) and compound III-3 (14.01g, 0.081mol, 1.0eq.) were dissolved in 200mL toluene, and NaBH was added in batches 3 After adding CN (19.17g, 0.146mol, 1.8eq.), the reaction was stirred at room temperature for 18h, and TLC showed that the reaction was complete. Slowly pour the reaction solution into 400mLNaHCO 3 Aqueous solution, stirred until no bubbles, extracted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com