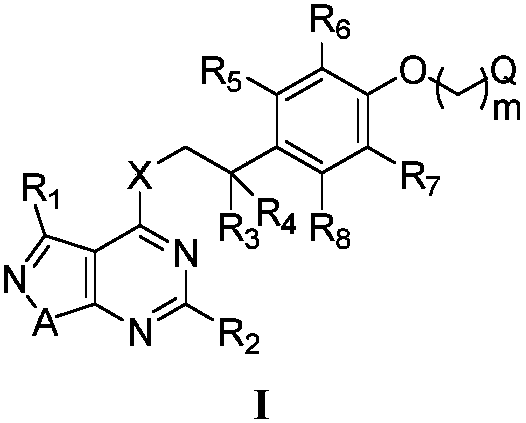
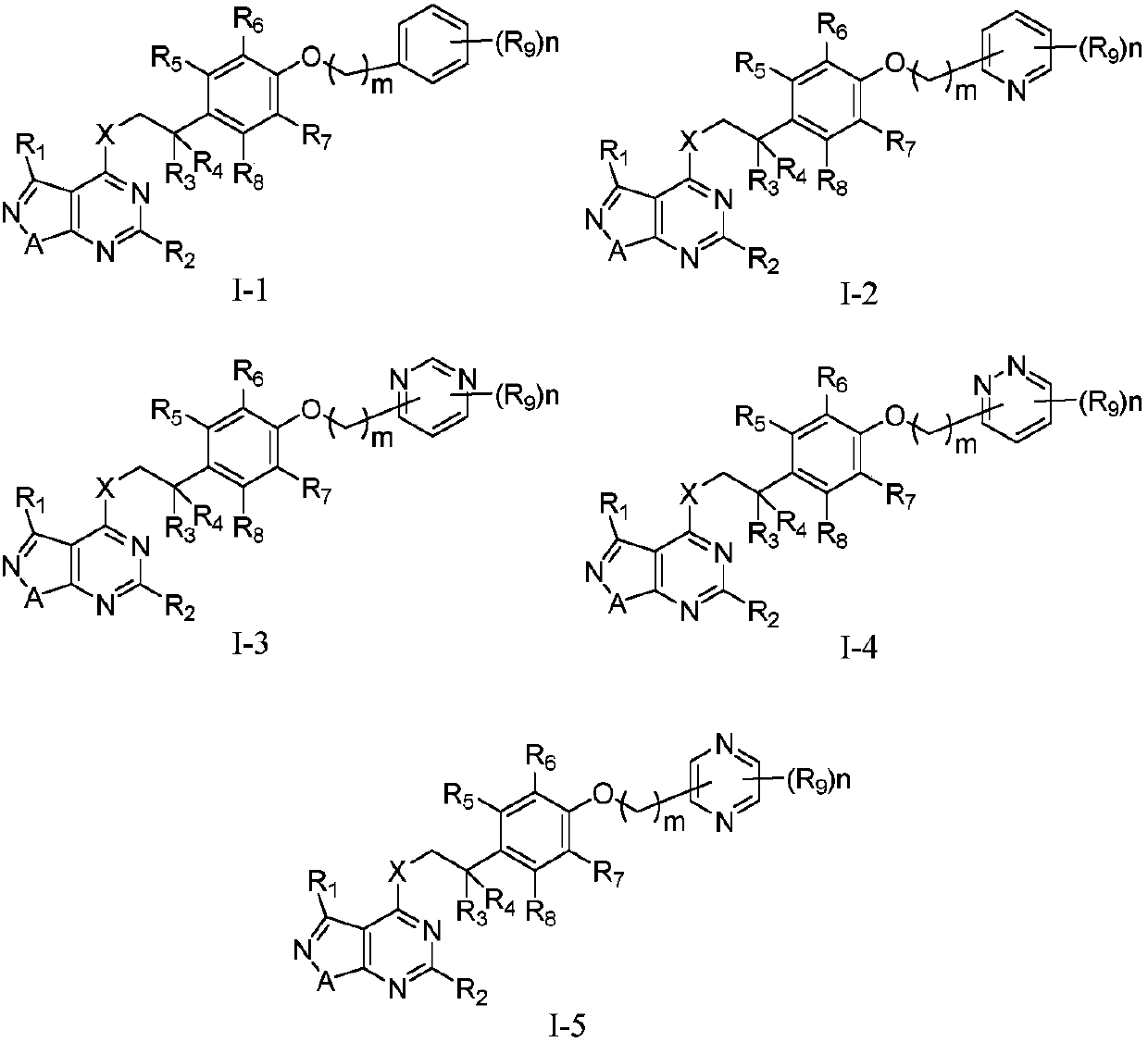
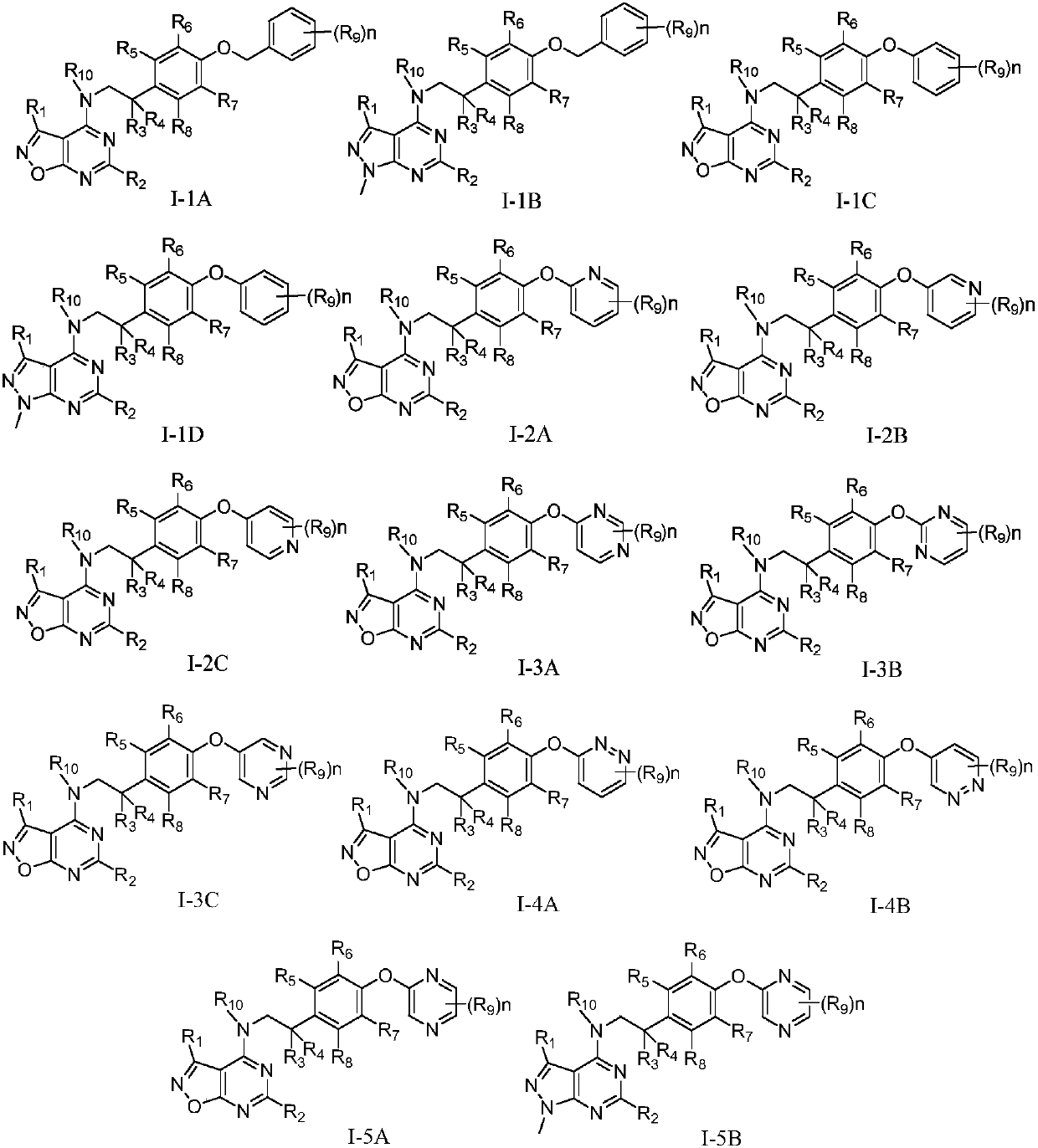
Pyrimidine-containing fused heterocyclic compounds, and preparation method and application thereof
A compound and cyclic technology, applied in the field of pyrimidocyclic compounds and their preparation, can solve the problems such as unreported structural pyrimidocyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0266] Embodiment 1: Preparation of intermediate 2-((3-methylisoxazolo[5,4-d]pyrimidin-4-yl)amino)ethanol
[0267] 1) Preparation of 5-amino-3-methylisoxazole-4-cyano
[0268]
[0269] Dissolve 14g (0.2 mol) of hydroxylamine hydrochloride in 80mL of 10% sodium hydroxide, add 27.2g (0.2 mol) of (1-ethoxyethylene) malononitrile, add a small amount of ice to keep the temperature below 50°C, and continue at room temperature After stirring for 1.5 hours, the solid was filtered, washed with water, and recrystallized from absolute ethanol to obtain 16.5 g of a solid product.
[0270] 2) Preparation of 4-chloro-3-methylisoxazolo[5,4-d]pyrimidine
[0271]
[0272] Phosphorus oxychloride (POCl 3 ) 40mL and N,N-dimethylformamide 1mL were added to the reaction flask, stirred at room temperature for 1 hour, added 5-amino-3-methylisoxazole-4-cyano group (451mg, 3.66mmol), and heated to React at 160°C for 15-36 hours, and distill the reaction solution under reduced pressure to obtai...
Embodiment 2
[0276] Embodiment 2: the preparation of compound 7-1
[0277]
[0278] 0.54 g (2 mmol) of 4-(2-((3-methylisoxazolo[5,4-d]pyrimidin-4-yl)amino)ethyl)phenol and 0.25 g (2 mmol) of benzyl chloride were added Add 0.41g (3mmol) of potassium carbonate to 5ml of N,N-dimethylformamide, heat to 80°C, and react for 4-10 hours. After the reaction is monitored by TLC, pour the reaction solution into 50ml of saturated saline, Extraction was carried out in three portions with 100 ml of ethyl acetate and dried. After precipitation, the product was purified by column chromatography to obtain 0.45 g of the product, namely compound 7-1, with a melting point of 123.0°C.
[0279] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm):8.50(s,1H),7.35-7.42(m,5H),7.15(d,2H),6.97(d,1H),5.16(s,1H),5.07(s,2H), 3.87(q,2H), 2.93(t,2H), 2.37(s,3H).
Embodiment 3
[0280] Embodiment 3: the preparation of compound 31-33
[0281]
[0282] Add 0.16 g of 60% sodium hydride into the reaction flask, wash with petroleum ether, add 5 ml of N,N-dimethylformamide, and then add 4-(2-((3-methylisoxazolo[ 5,4-d]pyrimidin-4-yl)amino)ethyl)phenol 0.54g (2mmol), after stirring at room temperature for 2 minutes, add intermediate 2,3-dichloro-5-trifluoromethylpyridine 0.43g (2mmol ), stirred and reacted at 60°C for 2 hours. After the reaction was monitored by TLC, the reaction solution was poured into 50 ml of saturated brine, extracted three times with 100 ml of ethyl acetate, and dried. After precipitation, 0.58 g of the product was purified by column chromatography, namely compound 31-33. The melting point is 141.7°C.
[0283] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm):8.53(s,1H),8.25(s,1H),7.99(s,1H),7.32(d,2H),7.16(d,2H),5.25(s,1H),3.96( q,2H), 3.05(t,2H), 2.48(s,3H).
[0284] Other compounds of the present invention can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com