Chimeric antigen receptor cell library carrying gene element combination, preparation and screening method and application thereof
A chimeric antigen receptor and gene element technology, applied in the fields of biomedical engineering technology and synthetic biology, can solve problems such as changes in screening direction, antigen disease treatment products, and inability to directly target unknown antigens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1. Fully synthetic murine chimeric antigen receptor T cell library
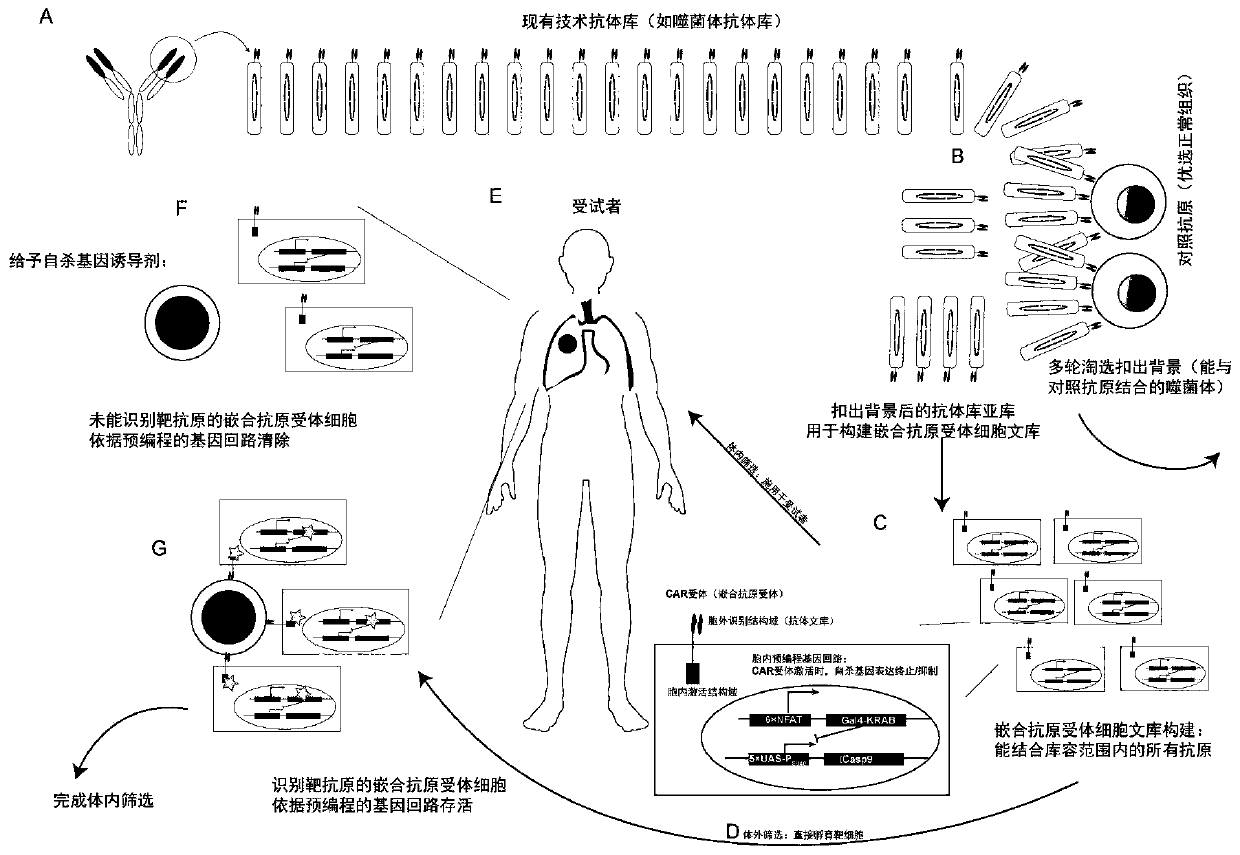
[0108] See the library construction and screening process figure 1 :
[0109] (A) Construction of phage antibody library: First, a fully synthetic murine phage single-chain antibody library is established using a total synthesis method. The method of antibody library preparation is well-known to those of ordinary skill in the art. The method of establishing a fully synthetic murine phage single-chain antibody library is the same as in the literature [Geuijen C et al.. European Journal of Cancer, 2005, 41(1): 178- 187; Noronha EJ, et al. Journal of Immunology, 1998, 161(6): 2968-2976.]. After the library capacity evaluation, the library capacity of the fully synthetic murine phage single-chain antibody library is 1×10 9 . The method of library capacity evaluation is the same as that in the literature [Ridgway J B B, et al. Cancer Research, 2013, 59(11): 2718-2723].
[0110] (B) Background eliminati...
Embodiment 2
[0119] Example 2. Treatment of breast cancer with fully synthetic mouse chimeric antigen receptor T cell library
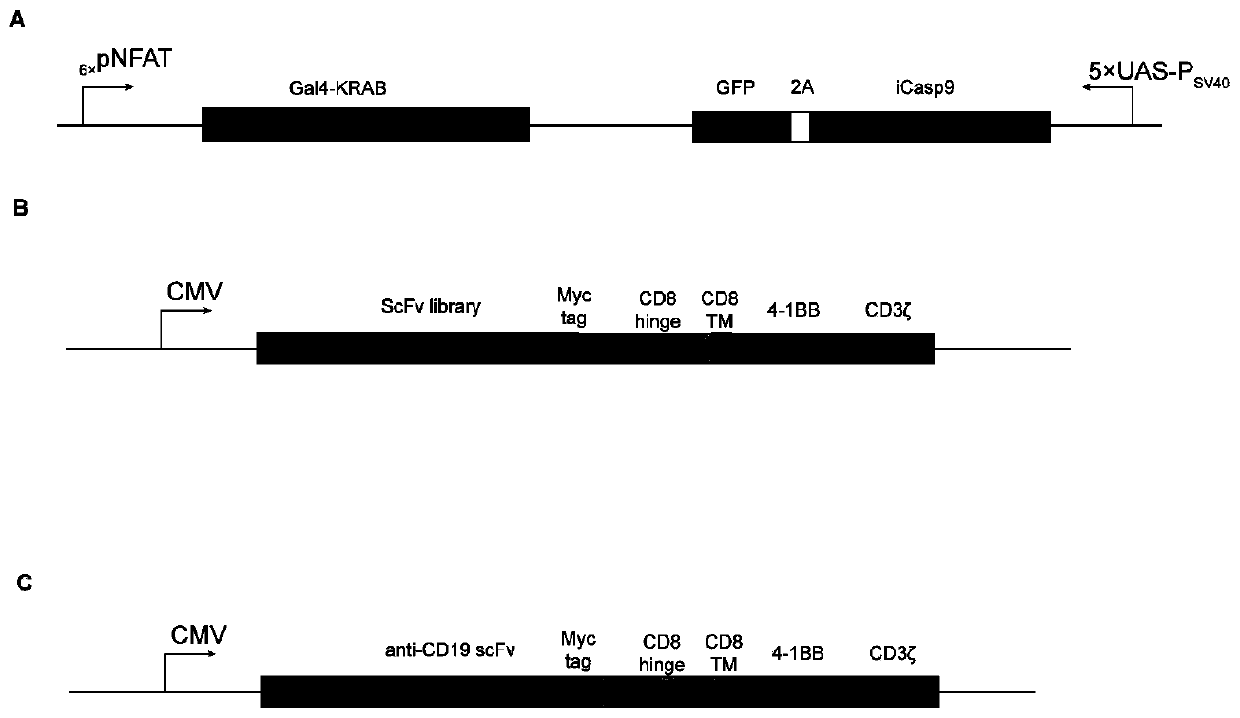
[0120] This example was performed using the KRAB-iCasp9-CAR-T cell library described in Example 1.
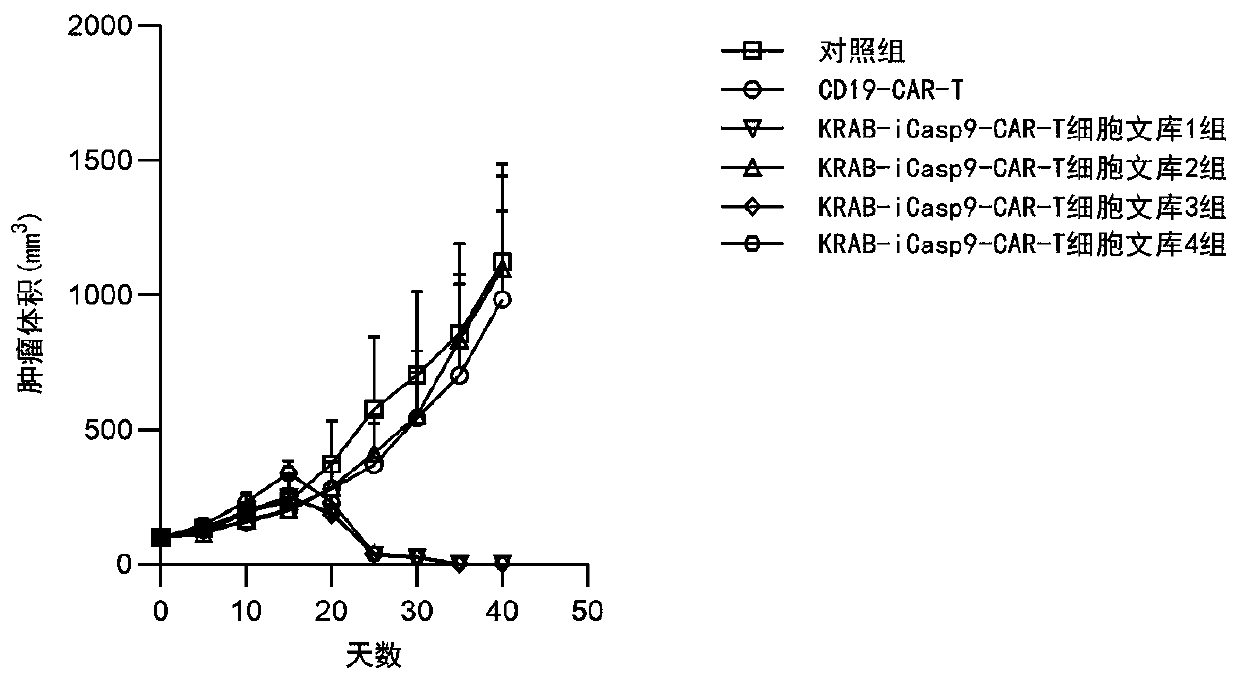
[0121] Establishment of 4T1 mouse orthotopic breast cancer model: The method of model establishment is the same as that in the literature [Paschall A V, Liu K. JoVE2016(114):e54040.]. When the tumor volume in mice reached an average of 100mm 3 Mice were divided into control group, unrelated CAR-T cell group, KRAB-iCasp9-CAR-T cell library group 1, KRAB-iCasp9-CAR-T cell library group 2, and KRAB-iCasp9-CAR-T cell library group 3. Group; 4 groups of KRAB-iCasp9-CAR-T cell library.
[0122] The CAR-positive rate of CAR-T cells is standardized to 45%. The control group was given PBS treatment; the unrelated CAR-T cell group was given CD19-CAR-T cell treatment (gene control expression cassette such as figure 2 C), the dose is 5×10 6 Cells were injected intravenously, once ...
Embodiment 3
[0124] Example 3. In vivo screening of fully synthetic murine chimeric antigen receptor T cells targeting 4T1 breast cancer
[0125] All the experimental groups in Example 2 were sacrificed after the experiment, and the blood of the mice was separated and the CAR-positive cells were detected by flow cytometry. Figure 4 shows the ratio of CAR-positive cells obtained after the experiment in all treatment groups. The CAR-positive cells in each group were separated and cultured to complete the in vivo screening.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com