Fetal-derived adult cholestatic liver injury animal model, its specific biomarkers and its application
A technology of cholestasis and animal models, applied in biochemical equipment and methods, compound screening/testing, preparations for in vivo experiments, etc., to achieve high repeatability, easy promotion and application, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] [Example 1] Construction of an animal model of fetal-derived adult cholestatic liver injury
[0035] Proceed as follows:
[0036] 1. Animals and Handling
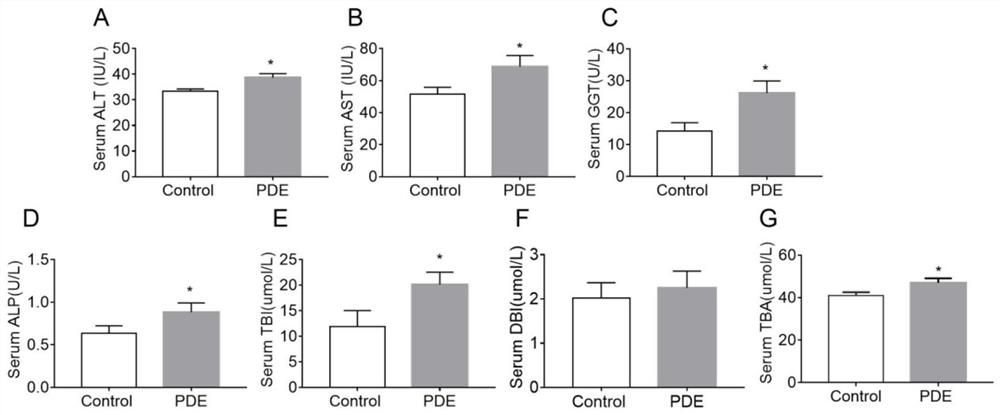
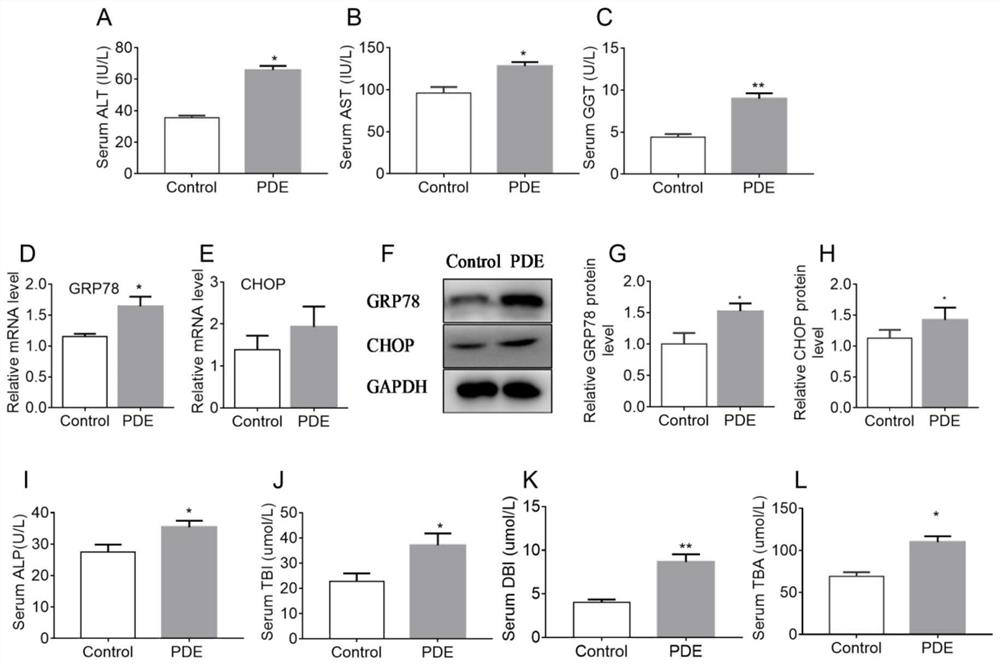
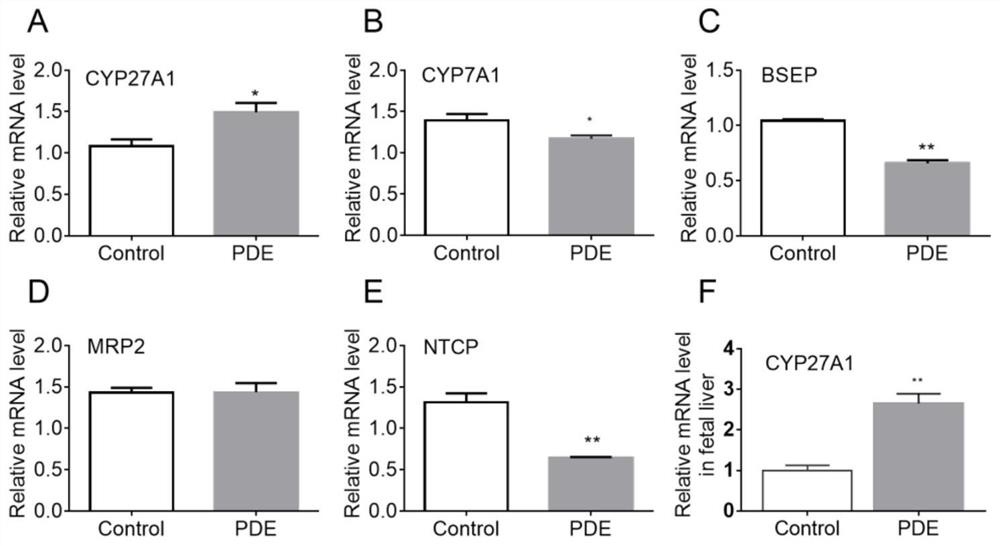
[0037] 20 male Wistar rats (body weight 260-300g), and 40 female Wistar rats (body weight 200-240g). Provided by Hubei Provincial Animal Center. Free to drink and eat, after 7 days of adaptive feeding, according to male: female = 1:2 cage, vaginal smear in the next morning, to determine the pregnant rats, recorded as pregnancy 0 day. The pregnant rats were randomly divided into two groups: the control group and the prenatal dexamethasone exposure (PDE) group, and the experimental group (PDE group) pregnant rats were treated with 0.2 mg / kg.d dexamethasone subcutaneously from GD9 to GD20. Injection treatment, while the control group was given the same volume of saline. Some pregnant mice were sacrificed on the 20th day of pregnancy to take the livers of the fetuses, and the relevant indicators of liver bile acid me...
Embodiment 2
[0063] [Example 2] Using the animal model of the present invention to screen for therapeutic drugs for fetal-derived adult cholestatic liver injury
[0064] Proceed as follows:
[0065] 1. Construct an animal model of fetal-derived adult cholestatic liver injury according to the method steps of Example 1;
[0066] 2. Give nilvadipine drug treatment to the fetal-derived adult cholestatic liver injury animal model constructed in step 1:
[0067] Select the female offspring of the PDE group, give the CYP27A1 activity inhibitor Nilvadipine (Nilvadipine) 1.4mg / kg.d from 8 weeks to 12 weeks after birth, and detect the effect of Nilvadipine on the cholestasis of the PDE group female offspring rats at 28 weeks effects of liver injury. The experiment was divided into four groups: the control group, which was not exposed to dexamethasone and given the same volume of normal saline; the PDE group; the Nilvadipine treatment group, which was given Nilvadipine drug intervention in the PDE ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com