Applications of bear gall dropping pills in preparation of antibacterial drugs
A technology of antibacterial drugs and dropping pills, applied in the field of biopharmaceuticals, to achieve the effect of inhibiting protein synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment one: the preparation of Xiongdan dripping pills
[0020] Xiongdan dripping pills were prepared according to the preparation method in the invention patent application 200410040574.8. The raw material bear bile powder used in the present invention is the dried bile of black bears or brown bears.
[0021] The present embodiment takes the preparation of Xiongdan dripping pills as an example, and the steps are as follows:
[0022] 1. Preparation of bear bile powder
[0023] Take the bear bile, dry it, crush it, and store it in a desiccator for future use.
[0024] 2. Preparation of Xiongdan Dropping Pills
[0025] (1) Add bear bile powder into melted polyethylene glycol 6000 or polyethylene glycol 4000 matrix, stir well, drop into drop pills, drain the coolant to obtain final product;
[0026] (2) The mechanisms used include but are not limited to polyethylene glycol 4000, polyethylene glycol 6000, sodium stearate, gelatin;
[0027] (3) The coolant used incl...
Embodiment 2
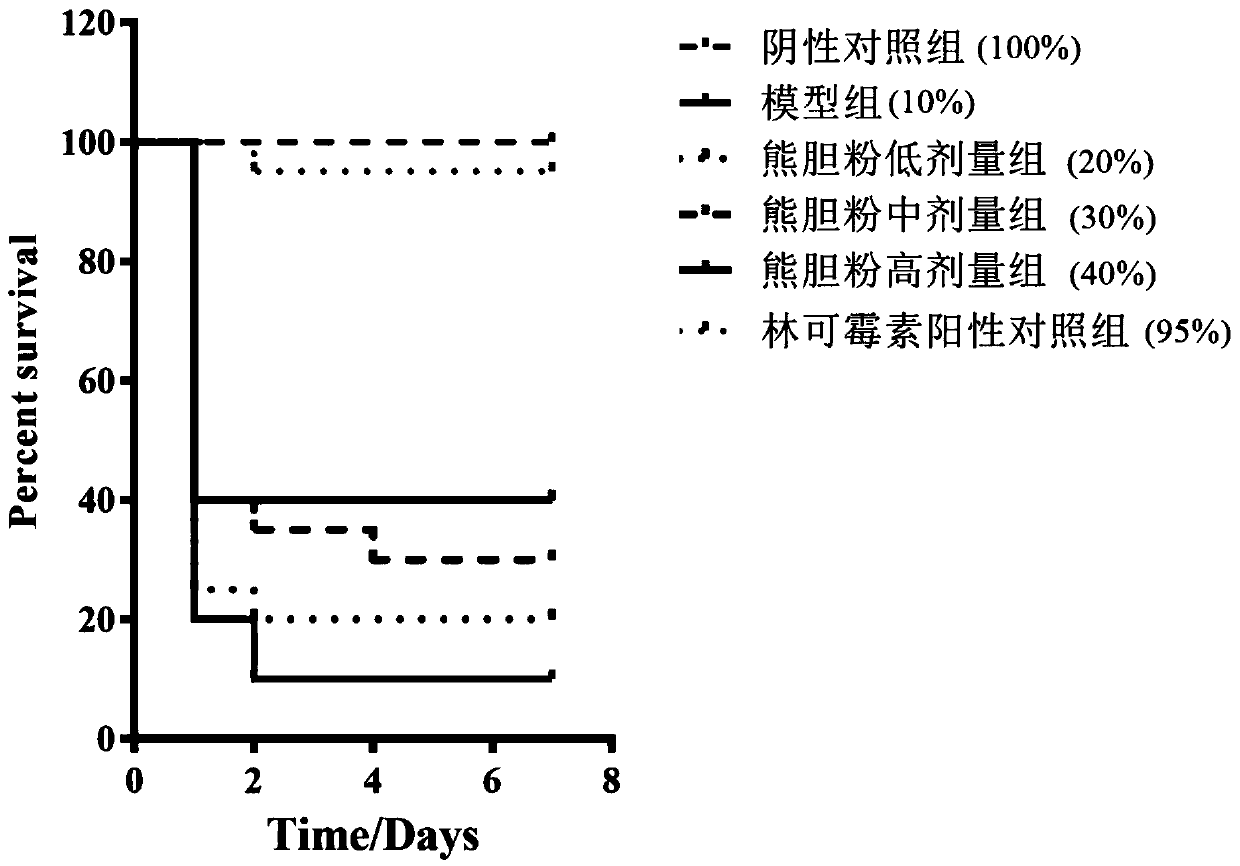
[0029] Example 2: Effect of Xiongdan Dripping Pills on Staphylococcus aureus-induced mouse infection model
[0030] 1. Materials
[0031] 1.1 Experimental animals
[0032] 1.1.1 Basic situation
[0033] (1) Animal strain: ICR mouse.
[0034] (2) Age: 4-6 weeks old.
[0035] (3) Gender: half male and half male
[0036] (4) Weight range: 18-24g.
[0037] (5) Source: Hunan Slack Jingda Experimental Animal Co., Ltd.
[0038] (6) Grade: SPF grade.
[0039] (7) Experimental animal production license number: SCXK (Xiang) 2016-0002 (valid until September 30, 2021), issued by Hunan Provincial Department of Science and Technology. Experimental animal quality certificate number: 43004700047690, 43004700048152, 43004700049697, 43004700051843.
[0040] 1.1.2 Animal grouping method:
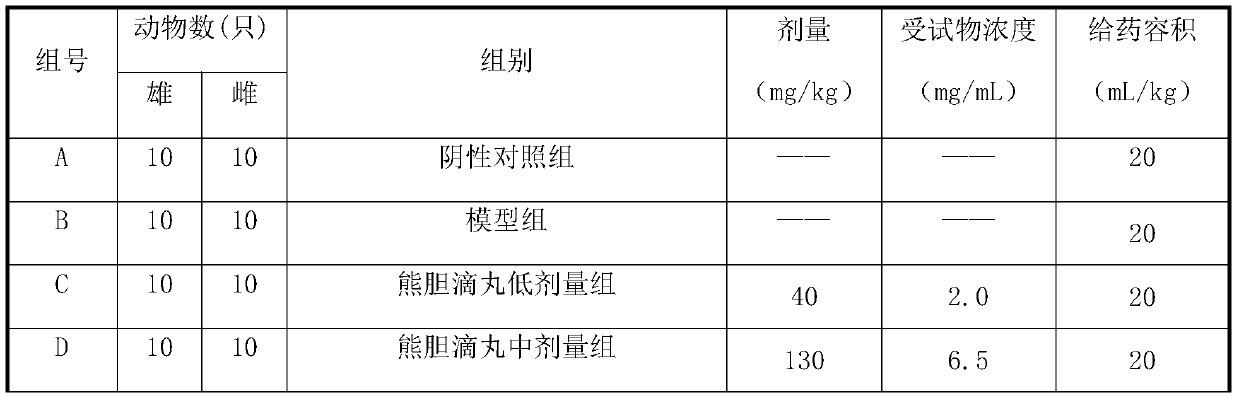
[0041] After the quarantine, the mice that passed the quarantine observation were selected and randomly divided into 6 groups according to their body weight and gender balance: negative control group,...
Embodiment 3
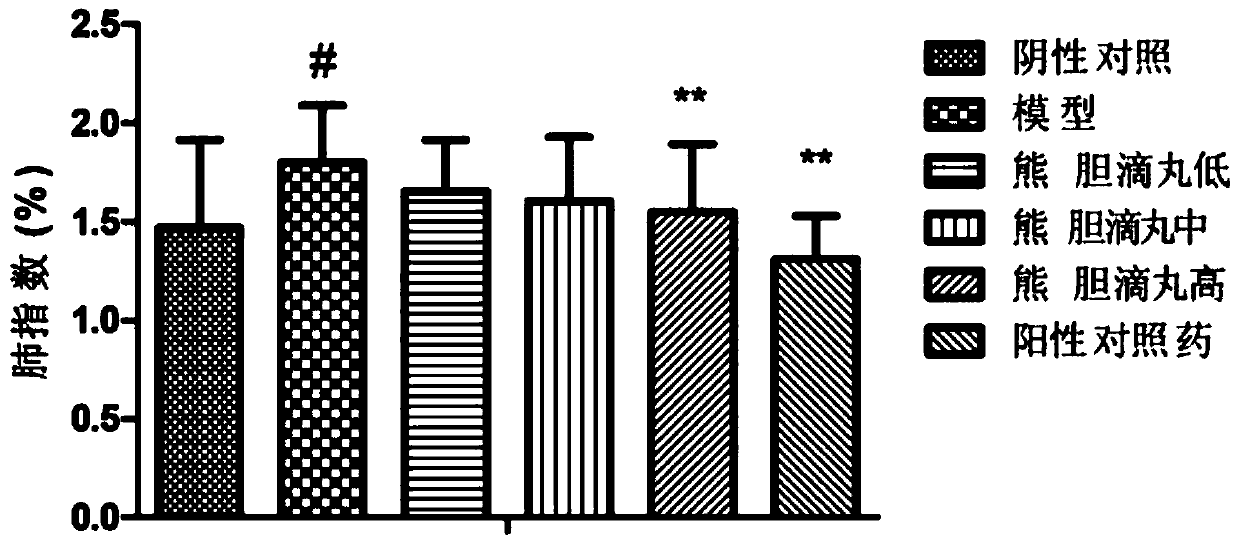
[0075] Example 3: Effect of Xiongdan Dropping Pills on Mouse Infection Model Caused by Streptococcus pneumoniae
[0076] 1. Materials
[0077] 1.1 Experimental animals
[0078] 1.1.1 Basic situation
[0079] (1) Animal strain: ICR mouse.
[0080] (2) Age: 4-6 weeks old.
[0081] (3) Gender: half male and half male
[0082] (4) Weight range: 18-24g.
[0083] (5) Source: Hunan Slack Jingda Experimental Animal Co., Ltd.
[0084] (6) Grade: SPF grade.
[0085] (7) Experimental animal production license number: SCXK (Xiang) 2016-0002 (valid until September 30, 2021), issued by Hunan Provincial Department of Science and Technology. Experimental animal quality certificate number: 43004700047690, 43004700048152, 43004700049697, 43004700051843.
[0086] 1.1.2 Animal grouping method:
[0087] After the quarantine, the mice that passed the quarantine observation were selected and randomly divided into 6 groups according to their body weight and gender balance: negative control g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com