Method for treating organic matter sewage through cooperation of ionic liquid and hydrogen peroxide
A technology of ionic liquid and hydrogen peroxide, which is applied in oxidized water/sewage treatment, chemical instruments and methods, water treatment of special compounds, etc., can solve problems such as high cost and unsuitable for large-scale sewage treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
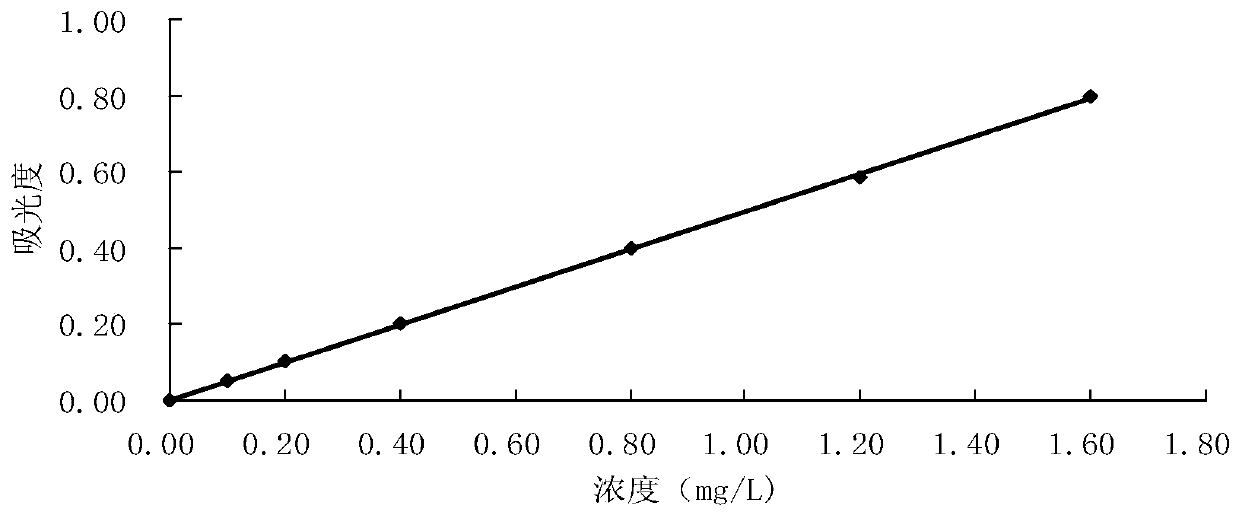
[0048] The drawing of embodiment 1 standard curve
[0049] Take seven 25mL volumetric flasks, add 0.00, 0.25, 0.50, 1.00, 2.00, 3.00, 4.00mL of aniline standard solution respectively, add water to 10mL, and shake well. Add 0.6 mL of 10% potassium bisulfate solution to adjust the pH to 1.5-2.0 (measured with precision pH test paper), add 1 drop of 5% sodium nitrite solution, shake well, and let stand for 3 minutes. Add 0.5 mL of 2.5% ammonium sulfamate solution, shake fully, and let stand for 3 min. After all the bubbles are removed, add 1.0mL of 2% NEDA solution, dilute to the mark with water, shake well, and let stand for 30min.
[0050] At a wavelength of 545mm, use a 10mm cuvette to measure the absorbance with water as a reference. Take the measured absorbance minus the absorbance of the blank test as the abscissa, and the corresponding aniline content as the ordinate to draw a standard curve, see figure 1 .
[0051] The obtained data is processed by regression, and the...
Embodiment 2
[0052] Calculation of embodiment 2 degradation rate
[0053] Take 30mL of aniline simulated sewage, add hydrogen peroxide and ionic liquid for degradation reaction. Take 0.4 mL of the reacted solution in a 25 mL volumetric flask, add water to 10 mL, and shake well. Add 0.6mL of 10% potassium bisulfate solution to adjust the pH to 1.5-2.0 (determined by precision pH test paper), add 1 drop of 5% sodium nitrite solution, shake well, and let stand for 3 minutes. Add 0.5 mL of 2.5% ammonium sulfamate solution, shake fully, and let stand for 3 min. After all the bubbles are removed, add 1.0mL of 2% NEDA solution, dilute to the mark with water, shake well, and let stand for 30min. At a wavelength of 545mm, measure the content of aniline in the water sample with a visible light spectrophotometer, and calculate the degradation rate as follows:
[0054]
[0055] in:
[0056] C 1 is the initial concentration of aniline sewage (mg / L);
[0057] C 2 is the concentration of anilin...
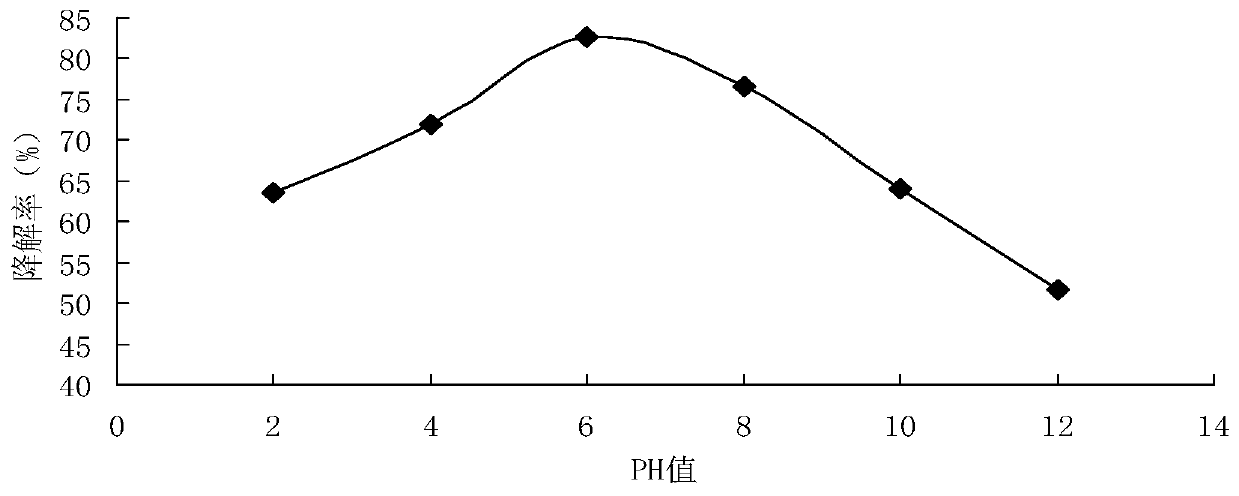
Embodiment 3
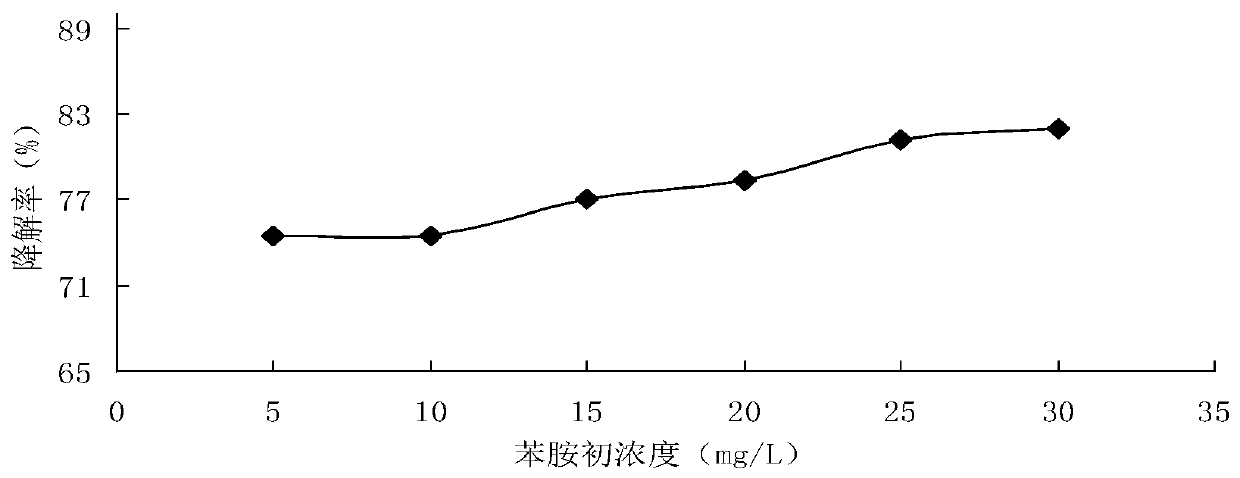
[0060] The influence of initial concentration on the degradation rate of aniline in the process of degrading aniline by hydrogen peroxide alone in embodiment 3
[0061] Prepare 30mL of aniline simulated sewage with initial concentrations of 5.0mg / L, 10.0mg / L, 15.0mg / L, 20.0mg / L, 25.0mg / L, and 30.0mg / L respectively, initial pH value of 6.0, hydrogen peroxide The amount of the added amount is 0.2mL, the reaction temperature is room temperature (about 18°C), and the reaction time is 30min. The degradation effect of aniline alone with hydrogen peroxide under different initial concentrations is figure 2 shown.
[0062] Depend on figure 2 It can be seen that the degradation rate of aniline increases with the increase of the initial concentration of aniline simulated sewage within the concentration range of 5.0mg / L-30.0mg / L, that is, a higher initial concentration is conducive to its degradation, and a lower initial concentration On the contrary, the degradation rate was low, but...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com