Thiophosphonate compound as well as preparation method and application thereof
A technology of thiophosphonate and compounds, which is applied in the field of thiophosphonate compounds, can solve the problem of inability to measure anti-wear performance and load-bearing capacity, inconsistent development trends, lack of extreme-pressure anti-wear agent load-bearing capacity and anti-wear properties. Grinding performance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
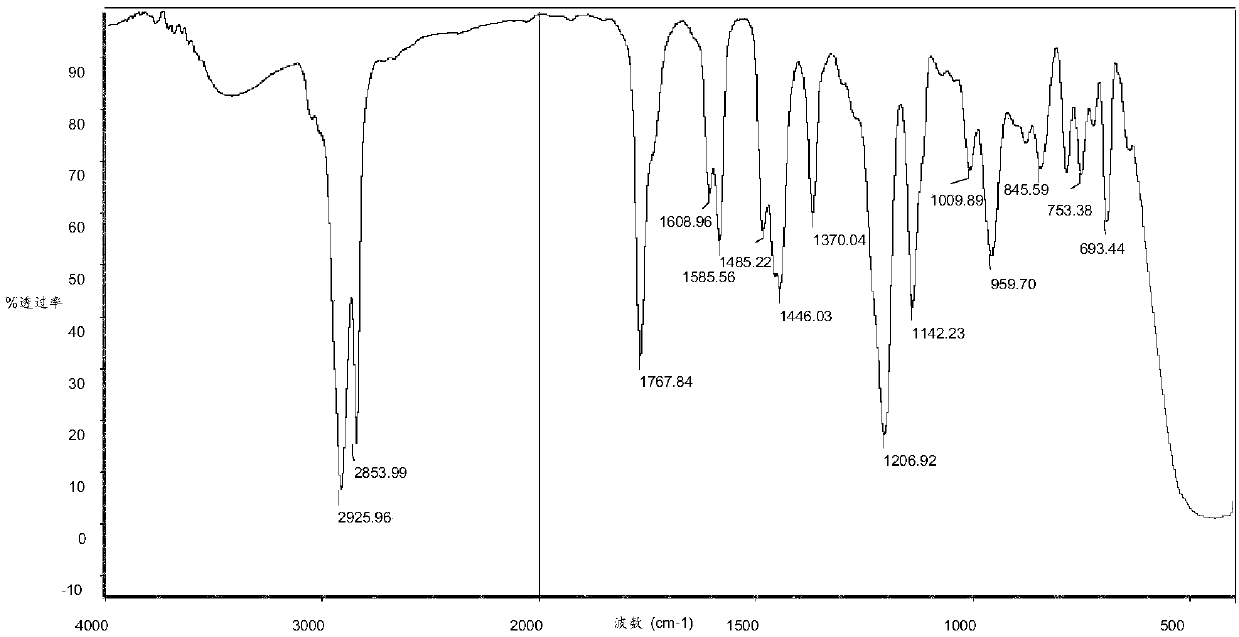
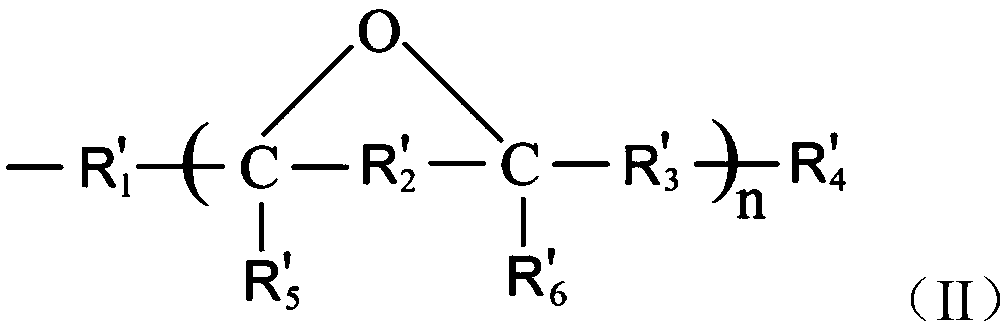
[0055] Example 1 Preparation of epoxidized cardanol
[0056] Take 100 g of cardanol, 8 g of formic acid, 0.3 g of sulfuric acid, and 200 g of hydrogen peroxide, add them to a three-necked flask with mechanical stirring, reflux condenser and temperature control, turn on stirring and heating. Maintain the reaction temperature at 70°C and react for 3 hours. After the reaction, the temperature was lowered to obtain a brown-red transparent liquid. The reaction product was filtered and washed with 5% KOH solution for alkaline washing, and then washed with distilled water to neutrality, and the organic phase was distilled under reduced pressure at 100Pa and 150℃ for 1h to remove water and unreacted raw materials to obtain orange-red transparent liquid . The product conversion rate is 96.2%, and the purity of the epoxidized cardanol is greater than 98%.
Embodiment 2
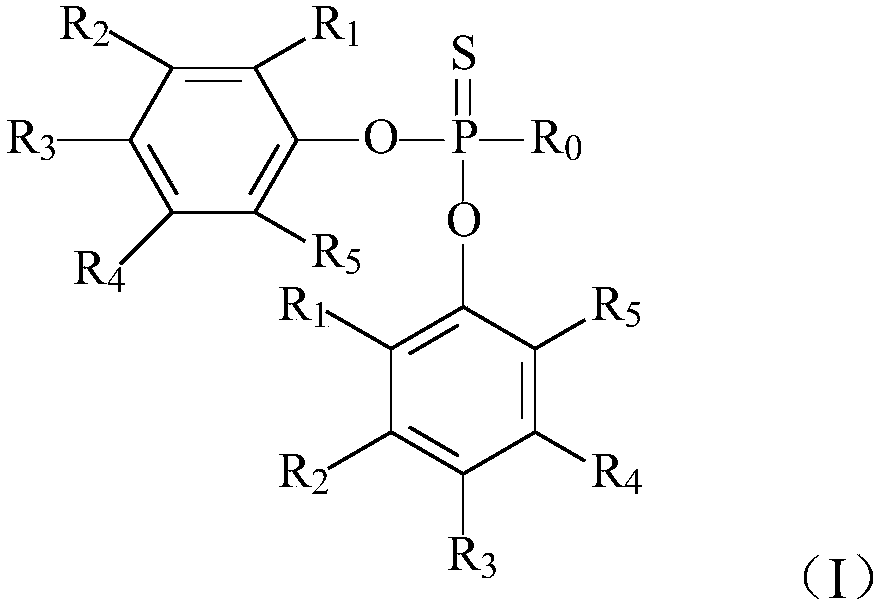
[0057] Example 2 Preparation of phenylthiophosphonic acid diepoxidized cardanol ester
[0058] Add 22g of epoxidized cardanol prepared in Example 1, 4g of triethylamine and 20g of toluene into the reaction flask, turn on heating and stirring, add 7.5g of phenylthiophosphonic dichloride, and maintain the reaction temperature at 70°C. React for 5 hours. After the reaction, the temperature was lowered to obtain a brown-red transparent liquid. The reaction product was washed with distilled water to neutrality, and the organic phase was distilled under reduced pressure at 100 Pa and 150° C. for 1 h to remove water and solvent to obtain a brown-yellow transparent liquid with a reaction conversion rate of 93.6%.
Embodiment 3
[0059] Example 3 Preparation of phenylthiophosphonic acid diepoxidized cardanol ester
[0060] Add 22g of the epoxidized cardanol prepared in Example 1, 8g of triethylamine and 50g of toluene into the reaction flask, turn on the heating and stirring, add 5g of phenylthiophosphonic dichloride, maintain the reaction temperature at 90°C, and react 4 hours. After the reaction, the temperature was lowered to obtain a brown-red transparent liquid. The reaction product was washed with distilled water until it was neutral, and the organic phase was distilled under reduced pressure at 100 Pa and 150° C. for 1 h to remove water and solvent to obtain a brown-yellow transparent liquid with a reaction conversion rate of 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com