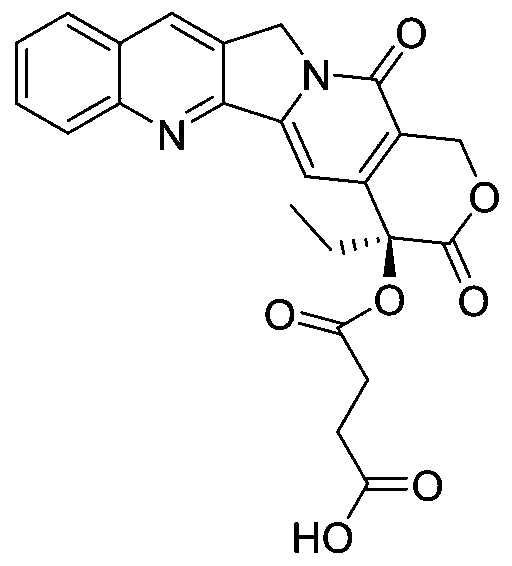
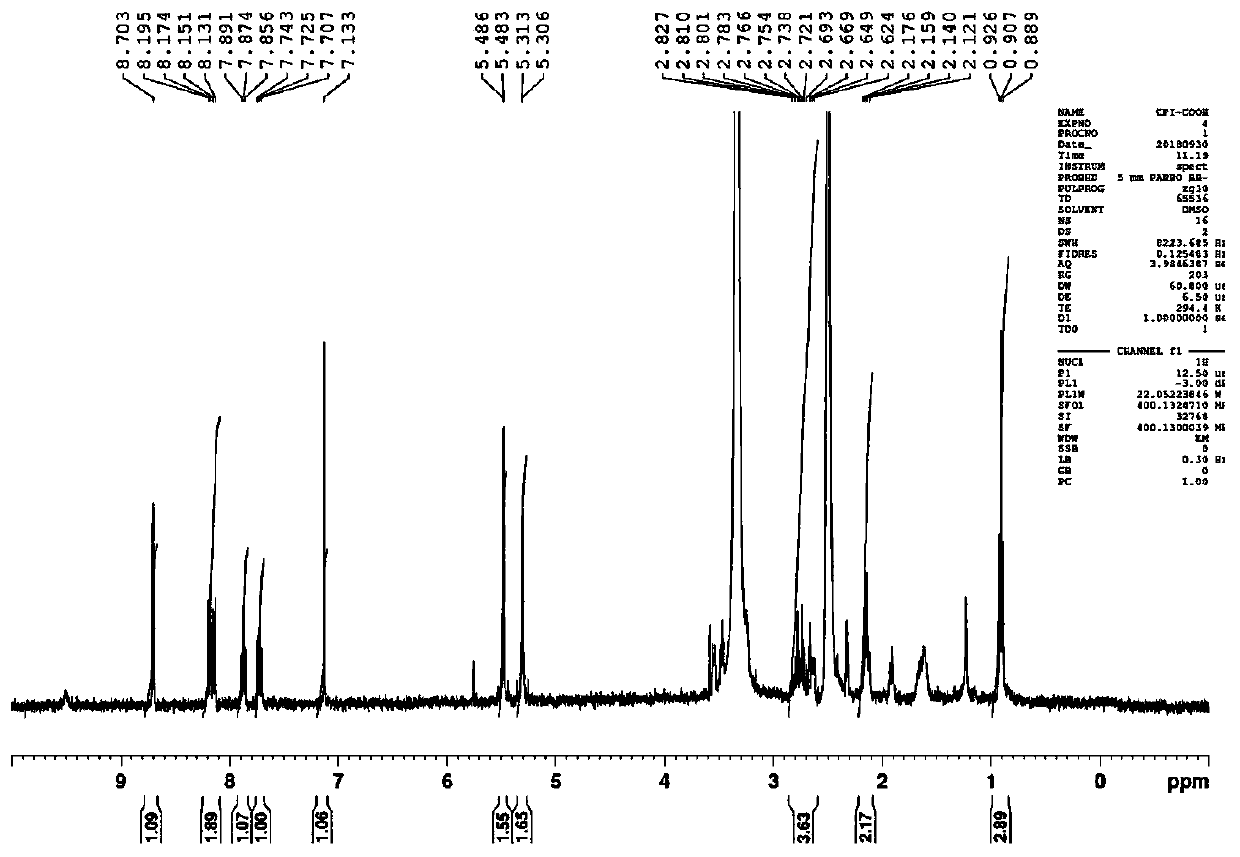
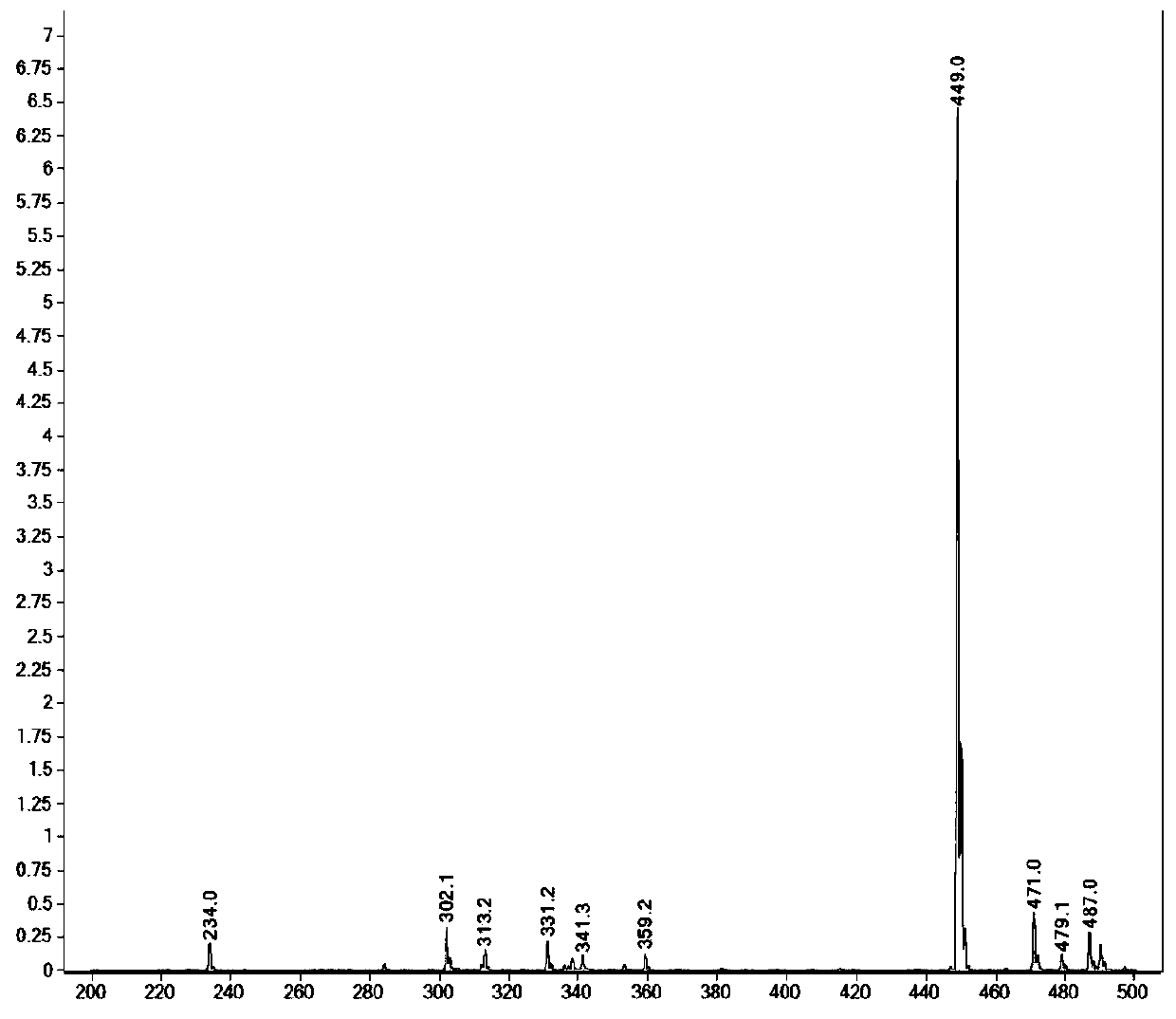
RGD polypeptide-camptothecin polypeptide drug conjugate and application thereof
A technology of drug conjugates and camptothecin, which is applied in the direction of drug combinations, antineoplastic drugs, and pharmaceutical formulations, can solve the problems of limiting camptothecin, achieve the effects of reducing biological toxicity, reducing accumulation, and improving antitumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation method of a RGD polypeptide-camptothecin-based polypeptide drug conjugate provided in this embodiment includes the following contents:
[0051] (1) Modification of camptothecin:
[0052] Using camptothecin and succinic acid dry as raw materials, DCM dehydrated as solvent, adding DBU to react at 10°C, after 4 hours, the reaction was completed, purified by methanol recrystallization to obtain camptothecin 20-OH modified product.
[0053] (2) Preparation of peptide drug conjugates of peptide-camptothecin
[0054](a) Rink amide resin was used as the starting material, soaked in DCM for 30 minutes, and then deprotected with piperidine / DMF; the ratio of Rink amide resin to DCM was 2:5.
[0055] (b) Dissolve Fmoc-Asp(OTBu)-OH and HBTU in DMF, connect with resin, and add DIEA to react; wherein the proportion of Fmoc-Asp(OTBu)-OH, HBTU, DMF is 1: 1:4, where DIEA is sufficient;
[0056] (c) Use piperidine / DMF to deprotect after the reaction;
[0057] (d) Fmoc...
Embodiment 2
[0061] The preparation method of a RGD polypeptide-camptothecin-based polypeptide drug conjugate provided in this embodiment includes the following contents:
[0062] (1) Modification of camptothecin:
[0063] Use camptothecin and succinic acid dry as raw materials, DCM with water removed as solvent, add DBU to react at 15°C, after 4 hours the reaction ends, purify with methanol recrystallization, and obtain camptothecin 20-OH modified product.
[0064] (2) Preparation of peptide drug conjugates of peptide-camptothecin
[0065] (a) Use Rink amide resin as the starting material, soak in DCM for 30 minutes, and then remove the protection with piperidine / DMF;
[0066] (b) Dissolving Fmoc-Asp(OTBu)-OH and HBTU in DMF, connecting with resin, and adding DIEA for reaction;
[0067] (c) Use piperidine / DMF to deprotect after the reaction;
[0068] (d) Fmoc-Gly-OH and Fmoc-Arg(pdf)-OH are connected according to the methods in (b) and (c) above;
[0069] (e) After the required amino...
Embodiment 3
[0075] The preparation method of a RGD polypeptide-camptothecin-based polypeptide drug conjugate provided in this embodiment includes the following contents:
[0076] (1) Modification of camptothecin:
[0077] Camptothecin and succinic acid were used as raw materials, DCM dehydrated as solvent, DBU was added to react at 0°C to room temperature, the reaction was completed after 4 hours, and purification was carried out by methanol recrystallization. The modified product of camptothecin 20-OH was obtained.
[0078] (2) Preparation of peptide drug conjugates of peptide-camptothecin
[0079] (a) Use Rink amide resin as the starting material, soak in DCM for 30 minutes, and then remove the protection with piperidine / DMF;
[0080] (b) Dissolving Fmoc-Asp(OTBu)-OH and HBTU in DMF, connecting with resin, and adding DIEA for reaction;
[0081] (c) Use piperidine / DMF to deprotect after the reaction;
[0082] (d) Fmoc-Gly-OH and Fmoc-Arg(pdf)-OH are connected according to the methods...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com