Non-peripheral quaternary ammonium group modified zinc phthalocyanine as well as preparation method and application thereof
A zinc phthalocyanine, non-peripheral technology, which is applied in the field of non-peripheral quaternary ammonium group modified zinc phthalocyanine and its preparation, can solve the problems of insufficient research on the structural characteristics of photosensitizers, lack of combined photosensitizers, clinical application limitations, etc. The effect of kinetic anticancer activity, clear structure, and improved tissue penetration ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
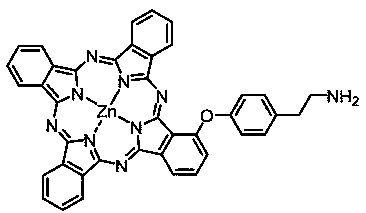
[0029] Non-peripheral quaternary ammonium group modified zinc phthalocyanine (1-[4-(N,N,N-trimethyl-2-aminoethyl)phenoxy] zinc phthalocyanine iodide), the structure is shown in the following formula:
[0030]
[0031] Weigh 20 mg (28.5 μmol) of 1-[4-(aminoethyl)phenoxy] zinc phthalocyanine and K 2 CO 3 (168.28 μmol) was dissolved in a single-neck round-bottom flask containing 10 ml of anhydrous DMF under ultrasound, and 2000 mg CH was slowly added after cooling to 0°C 3 I. After stirring for 30 min, react at room temperature. TLC spot plate, stop the reaction after 24 h, spin dry the reaction solvent, dissolve the reactant with 5 ml DMF and filter with a 0.22 μm syringe filter to remove insoluble matter. Vacuum and spin dry the solvent, dissolve it with 1ml DMF, pass the S-X1 gel column and use DMF as the eluent to collect the forefront blue-green components. Vacuum and spin dry the solvent, dissolve it with EA and pass it through a 100-200 mesh silica gel column (eluent is EA:DM...
Embodiment 2
[0034] The reaction solvent of Example 1 is replaced with 6ml or 60ml of anhydrous DMF, and other conditions remain unchanged, and the target product can also be obtained. The structural characterization data of the product are as follows: 1 H NMR (400 MHz, DMSO) δ 9.23 (d, J = 23.3 Hz, 6H), 8.83 (s, 1H), 8.16 (s, 6H), 7.77 (d, J = 6.2 Hz, 1H), 7.44 (s, 2H), 7.37 (s, 2H), 7.09 (s, 1H), 3.90 (s, 2H), 2.74 (s, 2H), 1.50 (s, 2H), 1.26 (s, 4H),0.84 (s, 3H). HRMS (ESI) m / z calcd for C 43 H 32 N 9 OZn [M-I] + : 754.2016; found:754.2042. HPLC (674 nm):> 95%...
Embodiment 3
[0036] The 2000mg CH of Example 1 3 I, replace with 1000mg CH 3 I or 4000mg CH 3 I, other conditions remain unchanged, the target product can also be obtained. The structural characterization data of the product are as follows: 1 H NMR (400 MHz, DMSO) δ 9.23 (d, J = 23.3Hz, 6H), 8.83 (s, 1H), 8.16 (s, 6H), 7.77 (d, J = 6.2 Hz, 1H), 7.44 (s, 2H), 7.37 (s, 2H), 7.09 (s, 1H), 3.90 (s, 2H), 2.74 (s, 2H), 1.50 (s, 2H), 1.26 (s, 4H), 0.84 (s, 3H). HRMS (ESI) m / z calcd for C 43 H 32 N 9 OZn [M-I] + : 754.2016;found: 754.2042. HPLC (674 nm):> 95%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com