Synthesis method and application of ruthenium (II) polypyridine metal complex with anti-tumor effect
A technology of metal complexes and synthesis methods, which can be used in antitumor drugs, ruthenium organic compounds, platinum group organic compounds, etc., and can solve problems such as restricted use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
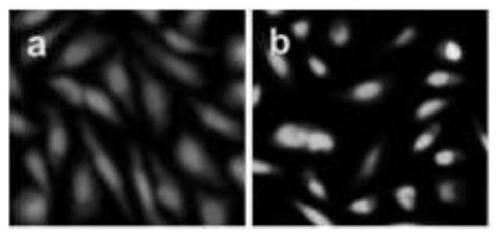
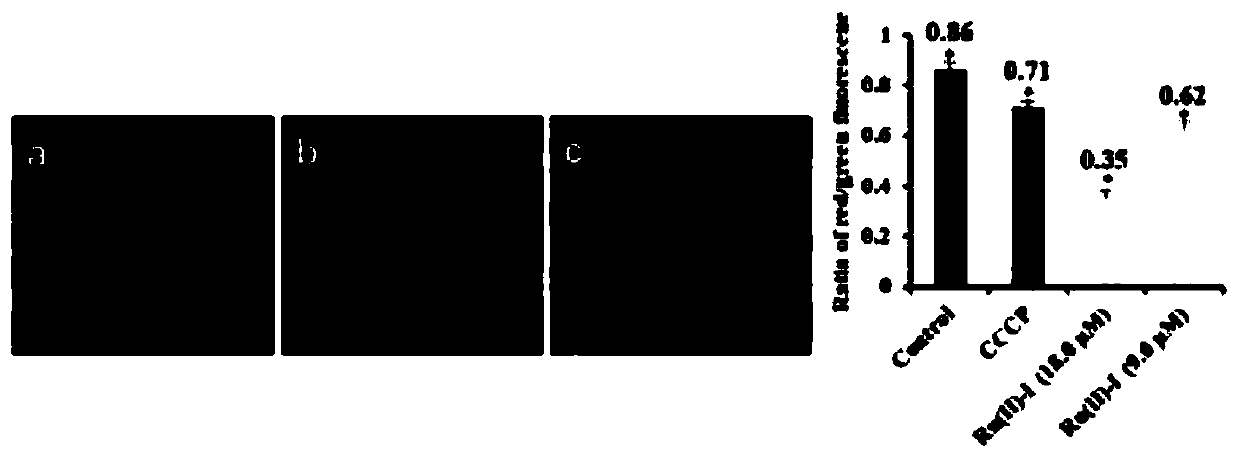
Examples
Embodiment 1
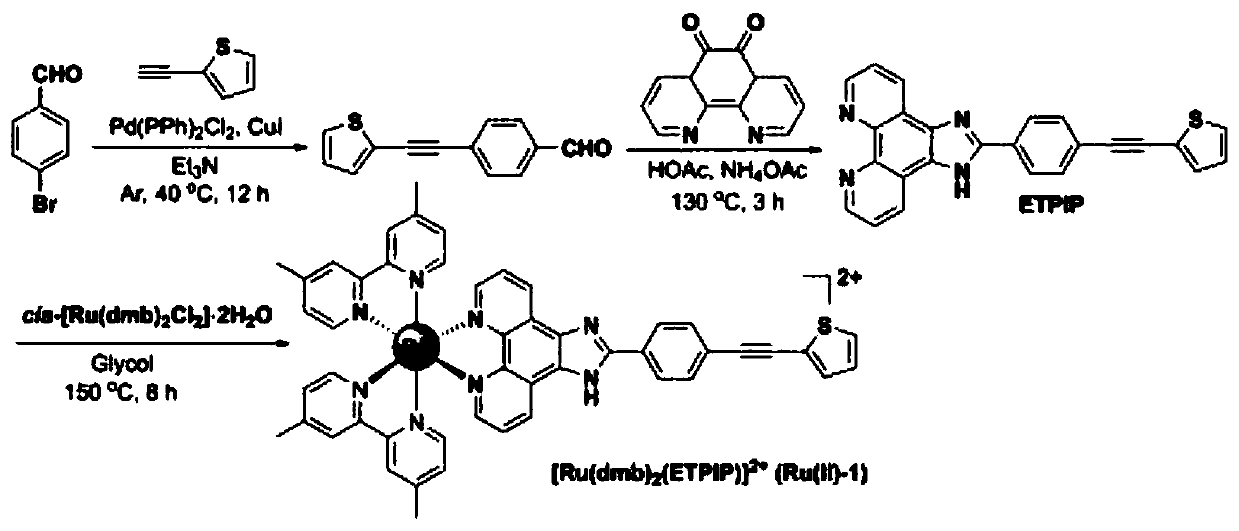
[0028] (1) Take a sealed tube of 25mL, add 1mmol of 4-bromobenzaldehyde, 1.3mmol of 2-ethynylthiophene, 0.1mmol of cuprous iodide, Pd(PPh 3 ) 2 Cl 2 0.05mmol and triethylamine 8mL toluene, under argon protection, stirred at 40°C for 8 hours (the extent of the reaction was detected by TLC), after the reaction was completed, cooled to room temperature and filtered, collected the filtrate, distilled under reduced pressure to remove triethylamine, passed through the column Purified by chromatography to obtain 4-(thiophene-2-ethynyl)benzaldehyde. The eluents used were petroleum ether and ethyl acetate, the volume ratio of which was petroleum ether:ethyl acetate=15:1, and the yield was 75%.
[0029] (2) Take a 150mL three-necked flask, add 1 mmol of 4-(thiophene-2-ethynyl)benzaldehyde, 1 mmol of o-phenanthroline-5,6-dione, 15 mmol of ammonium acetate and 30 mL of acetic acid in sequence, and stir the reaction at 130°C After 4 hours, cool to room temperature after the reaction, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com