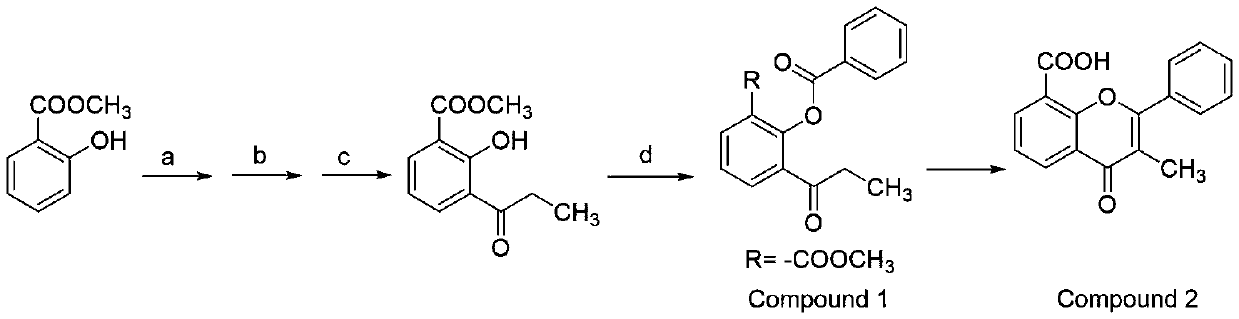
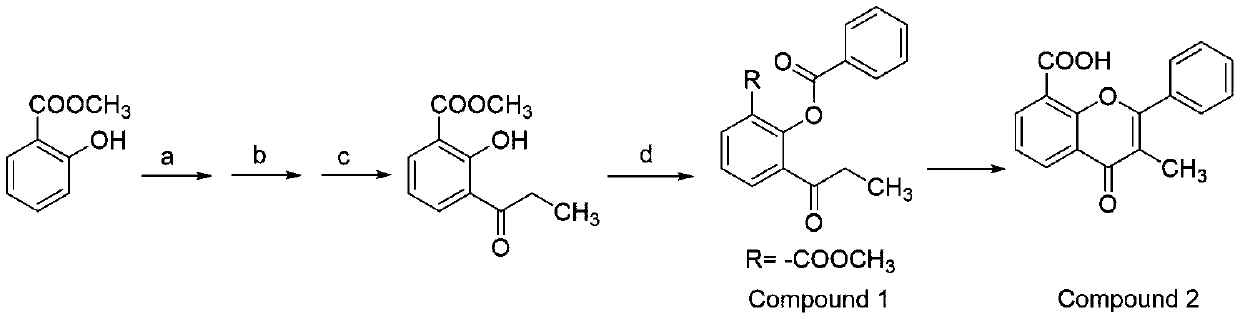
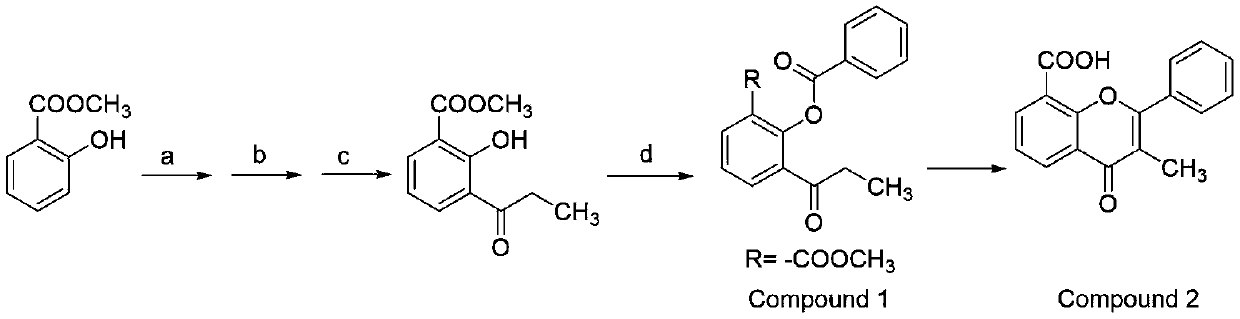
Preparation method for synthesizing flavoxate hydrochloride intermediate (3-methylflavone-8-carboxylic acid)
A technology of flavoxate hydrochloride and methylflavone, which is applied in the field of preparation of flavoxate hydrochloride intermediate 3-methylflavone-8-carboxylic acid, can solve the problems of difficult industrialization, waste of raw material processing, high conditions, etc. , to achieve the effect of reducing reaction steps, less waste, and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of compound 1:
[0025] a: bromine, dichloromethane, 0-15°C, 4 hours; b: propionyl chloride, aluminum trichloride, 60-65°C, 2.5 hours; c: hydrogen, palladium carbon, ethanol, room temperature, 1.5 hours. d: Benzoyl chloride, sodium hydroxide, dichloromethane, 0-25°C, 2-3 hours, extraction and separation, recovery of dichloromethane to give compound 1. 1 H NMR (600MHz, CDCl 3 )δ8.21 (2H, d, J = 7.32Hz), 8.18 (1H, d, J = 6.72Hz), 7.92 (1H, d, J = 7.74Hz), 7.65 (1H, t, J = 7.50Hz) ,7.53(2H,t,J=7.62Hz),7.42(1H,t,J=7.74Hz),3.73(3H,s),2.89(2H,q,J=6.96Hz),1.11(3H,t, J=7.20Hz); 13 C NMR (150 MHz, CDCl 3 )δ200.90,165.14,164.65,148.77,134.89,134.03,133.82,133.62,130.35, 129.18,128.75,126.03,124.79,52.44,35.72,8.22; MS m / z313.09(M+H + ).
[0026] Preparation of Compound 2:
[0027] Mix 320 grams of 2-benzoyloxy-3-propionylbenzoic acid methyl ester with 110 grams of dry basic alumina, start stirring, heat up the oil bath, control the temperature to 165°C, and tur...
Embodiment 2
[0031] Preparation of compound 1:
[0032] a: bromine, dichloromethane, 0-15°C, 4 hours; b: propionyl chloride, aluminum trichloride, 60-65°C, 2.5 hours; c: hydrogen, palladium carbon, ethanol, room temperature, 1.5 hours. d: Benzoyl chloride, sodium hydroxide, dichloromethane, 0-25°C, 2-3 hours.
[0033] Preparation of compound 2:
[0034] Mix 320 grams of 2-benzoyloxy-3-propionylbenzoic acid methyl ester with 110 grams of dry basic alumina, start stirring, heat up the oil bath, control the temperature to 175°C, and turn on the vacuum to remove water, and stir the reaction After 4.5 hours, the color of the reaction solution gradually changed to dark yellow, and the reaction was completed. Cool down to about 80°C, add 800ml of methanol, 115ml of 40% sodium hydroxide solution, control the reaction at 78°C for 1.5 hours, pH=11, cool down to 30°C, and filter Alumina is recovered and recycled after drying. The filtrate was adjusted to pH=3 with hydrochloric acid, crystallized f...
Embodiment 3
[0038] Preparation of Compound 1:
[0039] a: bromine, dichloromethane, 0-15°C, 4 hours; b: propionyl chloride, aluminum trichloride, 60-65°C, 2.5 hours; c: hydrogen, palladium carbon, ethanol, room temperature, 1.5 hours. d: Benzoyl chloride, sodium hydroxide, dichloromethane, 0-25°C, 2-3 hours.
[0040] Preparation of Compound 2:
[0041] Mix 320 grams of methyl 2-benzoyloxy-3-propionylbenzoate with 60 grams of dry basic alumina, start stirring, heat up the oil bath, control the temperature to 175°C, and turn on the vacuum to remove moisture, and stir the reaction After 5 hours, the color of the reaction solution gradually changed to dark yellow, and the reaction was completed. Cool down to about 80°C, add 800ml of methanol, 115ml of 40% sodium hydroxide solution, control the reaction at 80°C for 1.5 hours, pH = about 11, cool down to 30°C, and filter Alumina is recovered and recycled after drying. The filtrate was adjusted to pH=3 with hydrochloric acid, crystallized for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com