AgPdIr nano-alloy and preparation and use method thereof
A nano-alloy, nano-technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of low starting potential, low battery output voltage, low fuel conversion efficiency, etc., to improve catalytic activity and stability, improve Effects of Discharge Voltage and Energy Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
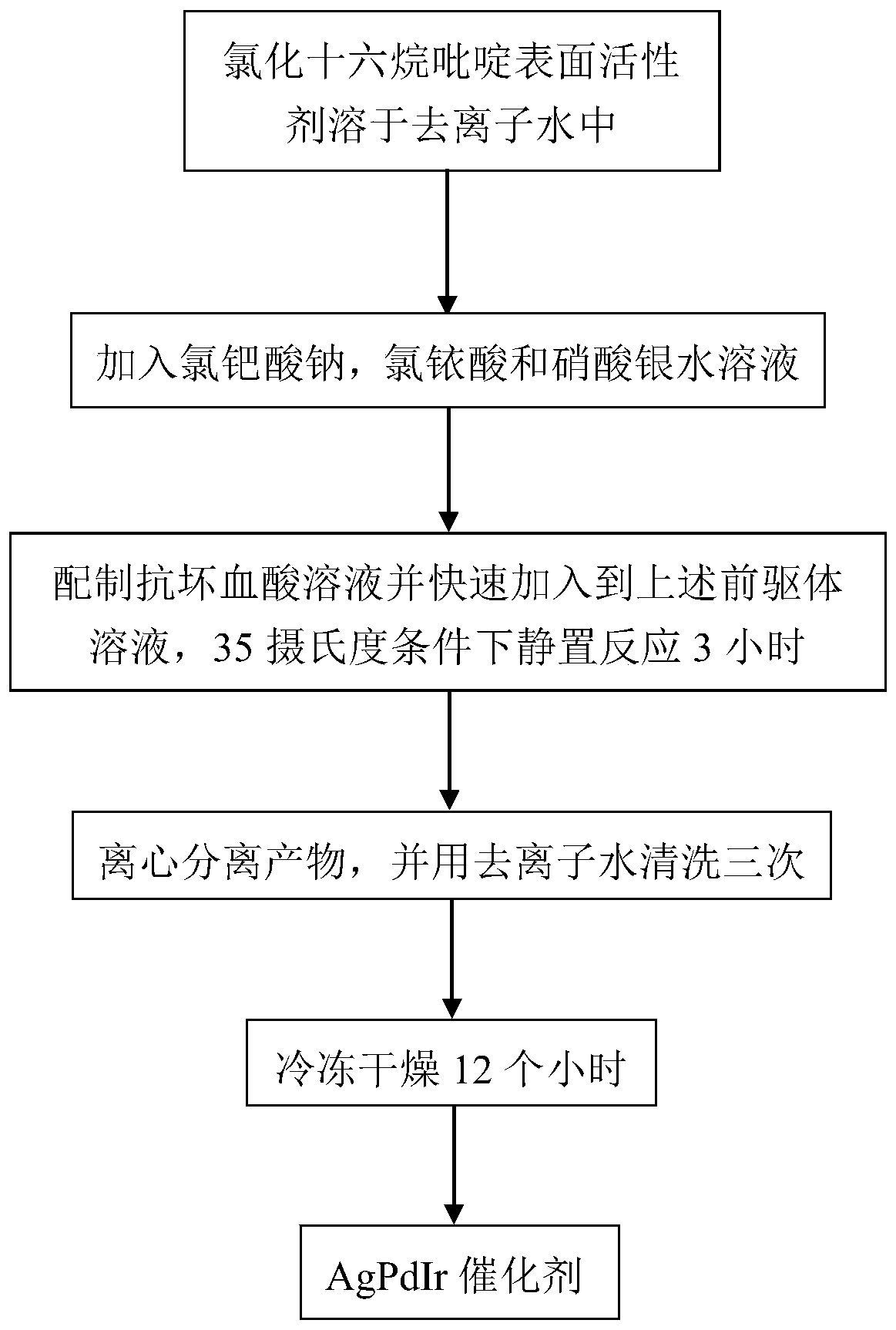
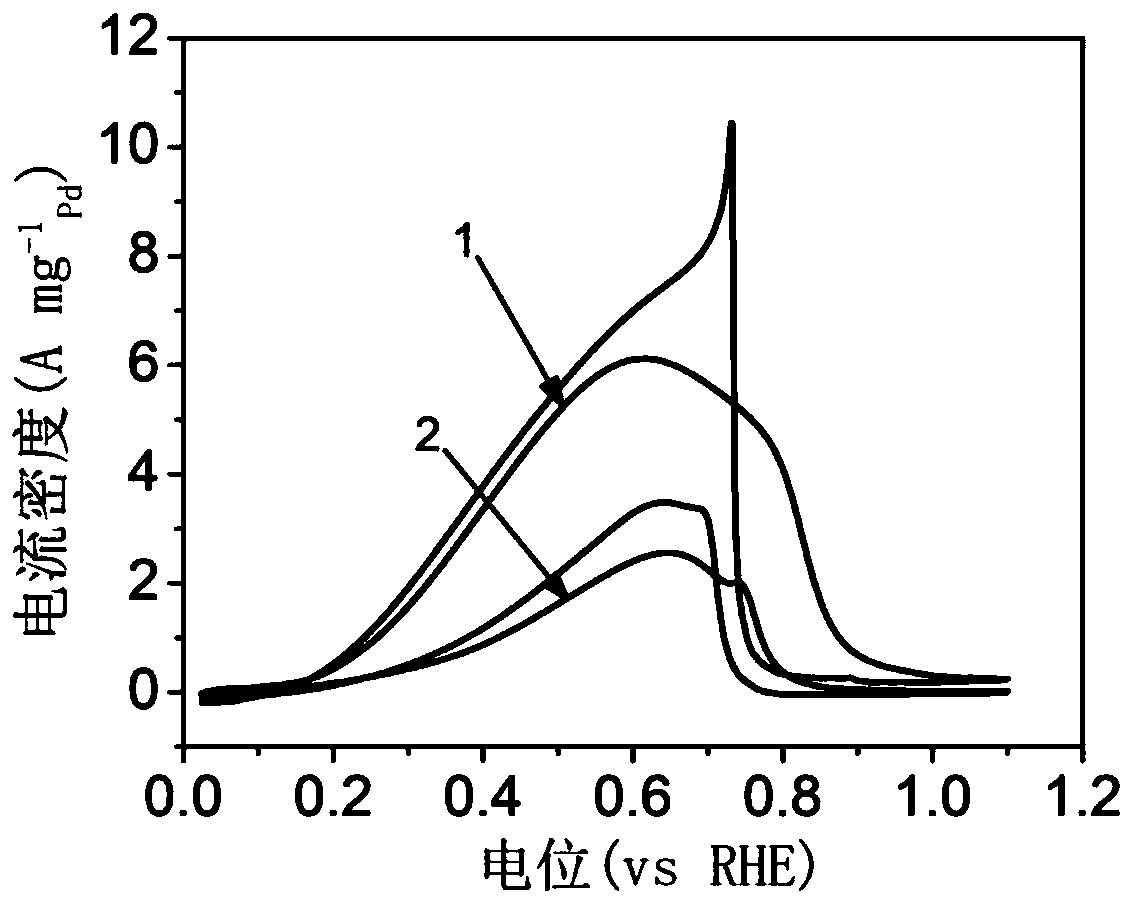
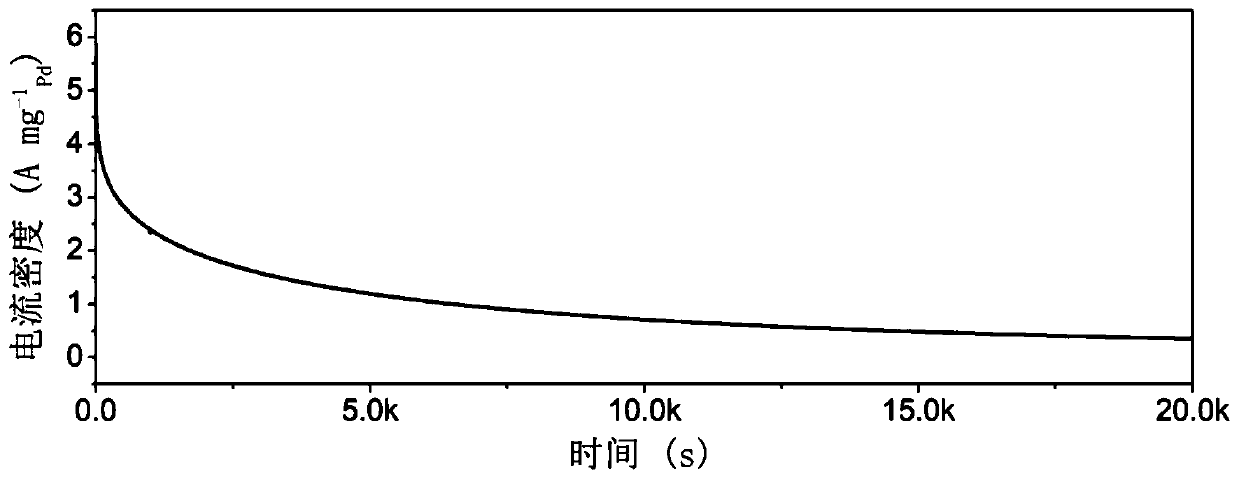
[0035] 18 mg of cetylpyridinium chloride surfactant was dissolved in 5 mL of deionized water, ultrasonicated for 10 minutes, and stirred for 10 minutes to form cetylpyridinium chloride aqueous solution. Take 0.267mL sodium chloropalladate (0.01mol·L -1 ), 0.12mL chloroiridic acid (0.01mol L -1 ) and 0.13mL silver nitrate (0.01mol·L -1 ) aqueous solution was added dropwise to the cetylpyridinium chloride aqueous solution obtained above, and stirred for 10 minutes to form an aqueous precursor solution. Prepare 0.3mL with a concentration of 0.1mol L -1 Ascorbic acid aqueous solution, and quickly added dropwise to the precursor aqueous solution obtained above, stopped stirring, and left to react at 35° C. for 3 hours. After the reaction is completed, the obtained black solution is centrifuged and washed three times with deionized water, and finally freeze-dried for 12 hours to obtain the final Ag 32 PD 66 Ir 2 Ternary nano-alloy catalyst. in N 2 The electrochemical test re...
Embodiment 2
[0037] 18 mg of cetylpyridinium chloride surfactant was dissolved in 5 mL of deionized water, ultrasonicated for 10 minutes, and stirred for 10 minutes to form cetylpyridinium chloride aqueous solution. Take 0.267mL sodium chloropalladate (0.01mol·L -1 ), 0.2mL chloroiridic acid (0.01mol L -1 ) and 0.13mL silver nitrate (0.01mol·L -1 ) aqueous solution was added dropwise to the cetylpyridinium chloride aqueous solution obtained above, and stirred for 10 minutes to form an aqueous precursor solution. Prepare 0.3mL with a concentration of 0.1mol L -1 Ascorbic acid aqueous solution, and quickly added dropwise to the precursor aqueous solution obtained above, stopped stirring, and left to react at 35° C. for 3 hours. After the reaction is completed, the obtained black solution is centrifuged and washed three times with deionized water, and finally freeze-dried for 12 hours to obtain the final Ag 30 PD 66 Ir 4 Ternary nano-alloy catalyst. in N 2 The electrochemical test res...
Embodiment 3
[0039] 18 mg of cetylpyridinium chloride surfactant was dissolved in 5 mL of deionized water, ultrasonicated for 10 minutes, and stirred for 10 minutes to form cetylpyridinium chloride aqueous solution. Take 0.267mL sodium chloropalladate (0.01mol·L -1 ), 0.32mL chloroiridic acid (0.01mol L -1 ) and 0.13mL silver nitrate (0.01mol·L -1 ) aqueous solution was added dropwise to the cetylpyridinium chloride aqueous solution obtained above, and stirred for 10 minutes to form an aqueous precursor solution. Prepare 0.3mL with a concentration of 0.1mol L -1 Ascorbic acid aqueous solution, and quickly added dropwise to the precursor aqueous solution obtained above, stopped stirring, and left to react at 35° C. for 3 hours. After the reaction is completed, the obtained black solution is centrifuged and washed three times with deionized water, and finally freeze-dried for 12 hours to obtain the final Ag 28 PD 65 Ir 7 Ternary nano-alloy catalyst. in N 2 The electrochemical test re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com