Production method and production device of metal halide
A metal halide and production method technology, applied in the field of materials, can solve the problems of highly toxic hazards to human body and the environment, harsh reaction conditions, and high preparation costs, and achieve the effects of easy separation and collection, sufficient reaction, and easy storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
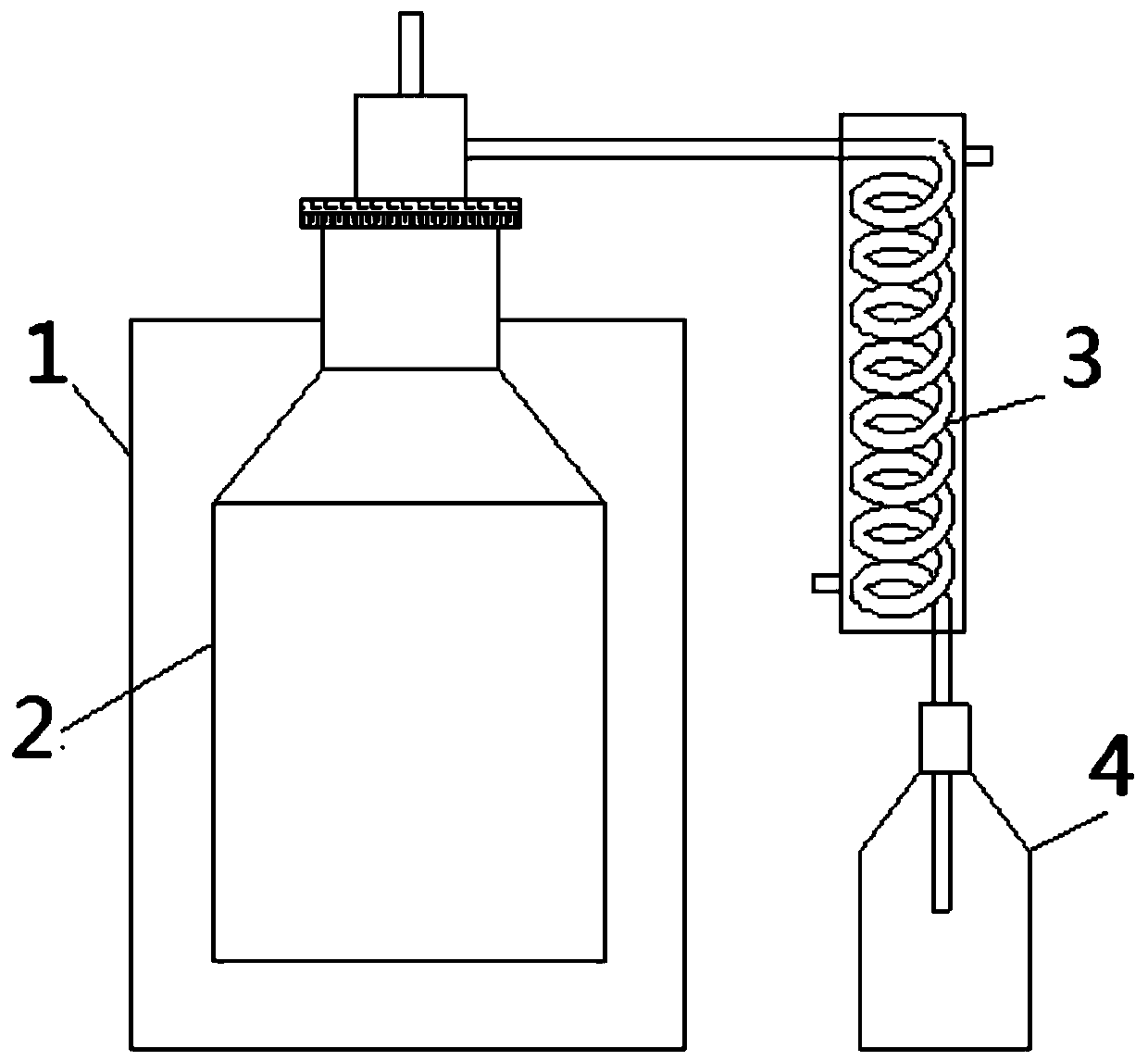
[0040] This embodiment discloses a kind of AlCl 3 The production method of the production method, the production device structure of the metal halide that this production method adopts is as follows image 3As shown, it includes a heating furnace 1, a reactor 2, a trap 3 and a collection container 4. The reactor 3 is arranged in the heating furnace 1, and the top outlet of the reactor 2 communicates with the collection container 4 through the trap 3.
[0041] AlCl 3 The production method comprises the following steps:
[0042] 1. Weigh aluminum phosphate and calcium chloride for grinding so that the molar ratio of aluminum to chlorine is 1:3;
[0043] 2. Place in a quartz reactor, then place the quartz reactor in a heating furnace, pass protective gas into the reactor for 1-5 hours; roast under protective gas conditions, keep the temperature at 250°C for 5 hours;
[0044] 3. Continue to heat up and roast under nitrogen atmosphere, the roasting temperature is 700° C., and th...
Embodiment 2
[0047] This embodiment discloses a ZnCl 2 The production method, the production device structure of the metal halide that this production method adopts is identical with embodiment 1.
[0048] ZnCl 2 The production method comprises the following steps:
[0049] 1. Weigh zinc silicate and sodium chloride for grinding and mixing, so that the molar ratio of vanadium to chloride is 1:2;
[0050] 2. Place in a quartz reactor, then place the reactor in a heating furnace, and pass protective gas into the reactor for 1-5 hours; roast under protective atmosphere conditions (argon, nitrogen, oxygen) , 200 ℃ constant temperature 6h;
[0051] 3. The obtained sample is roasted under an oxygen atmosphere, the roasting temperature is 650° C., and the roasting time is 4 hours. The residue in the collection bottle is the target product zinc chloride.
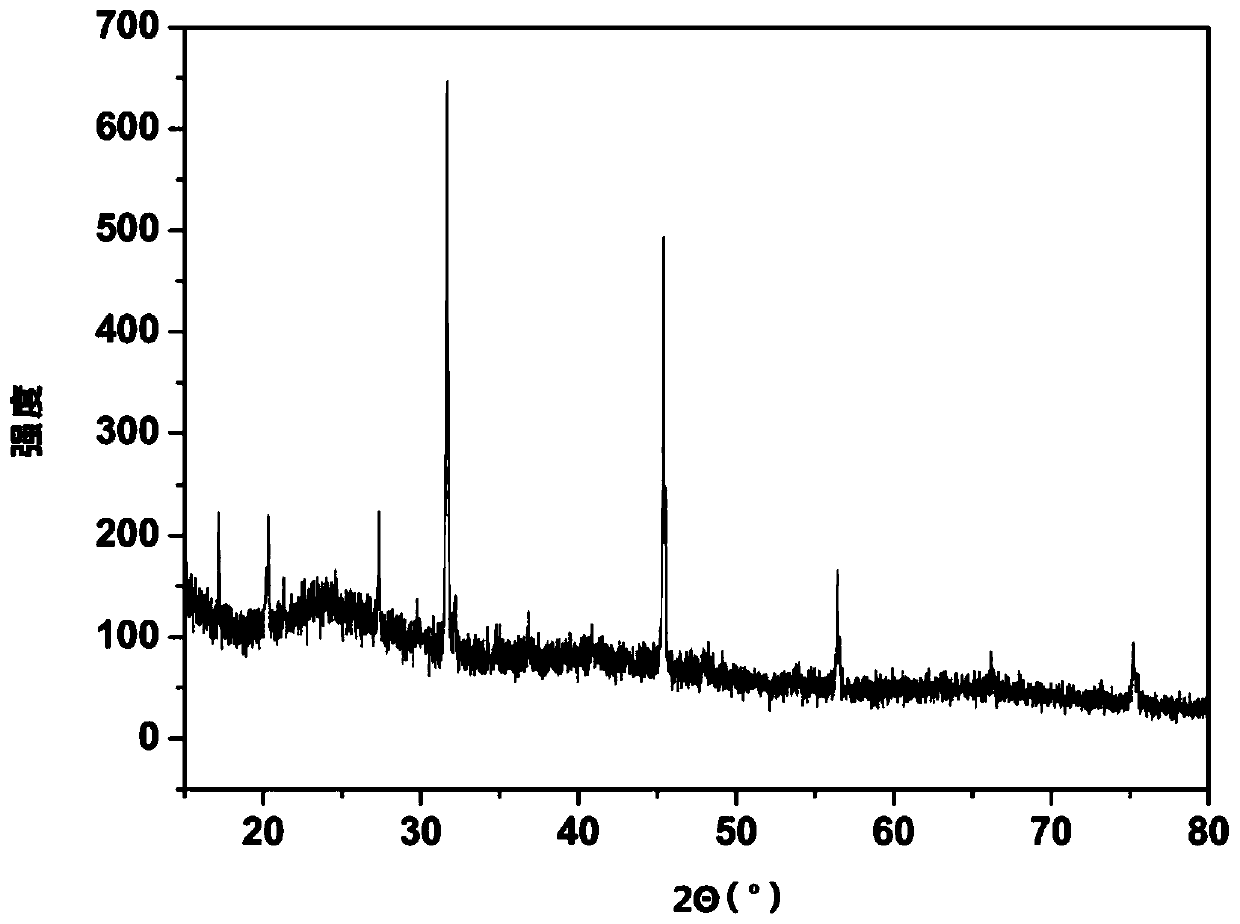
[0052] The ZnCl that present embodiment prepares 2 XRD characterization drawings are as follows image 3 As shown, the highest peak inte...
Embodiment 3
[0054] This embodiment discloses a TiCl 4 The production method, the production device structure of the metal halide that this production method adopts is identical with embodiment 1.
[0055] TiCl 4 The production method comprises the following steps:
[0056] 1. Weigh titanium dioxide and sodium chloride for grinding and mixing, so that the molar ratio of titanium to chloride is 3:4;
[0057] 2. Place it in a quartz reactor, then place the reactor in a heating furnace, and pass protective gas into the reactor for 1-5 hours; roast it under the condition of argon atmosphere, and keep the temperature at 300°C for 3 hours;
[0058] 3. The obtained sample was placed in a tube furnace and roasted under an argon atmosphere at a temperature of 850° C. for 3 hours. The residue in the collection bottle was the target product titanium tetrachloride.
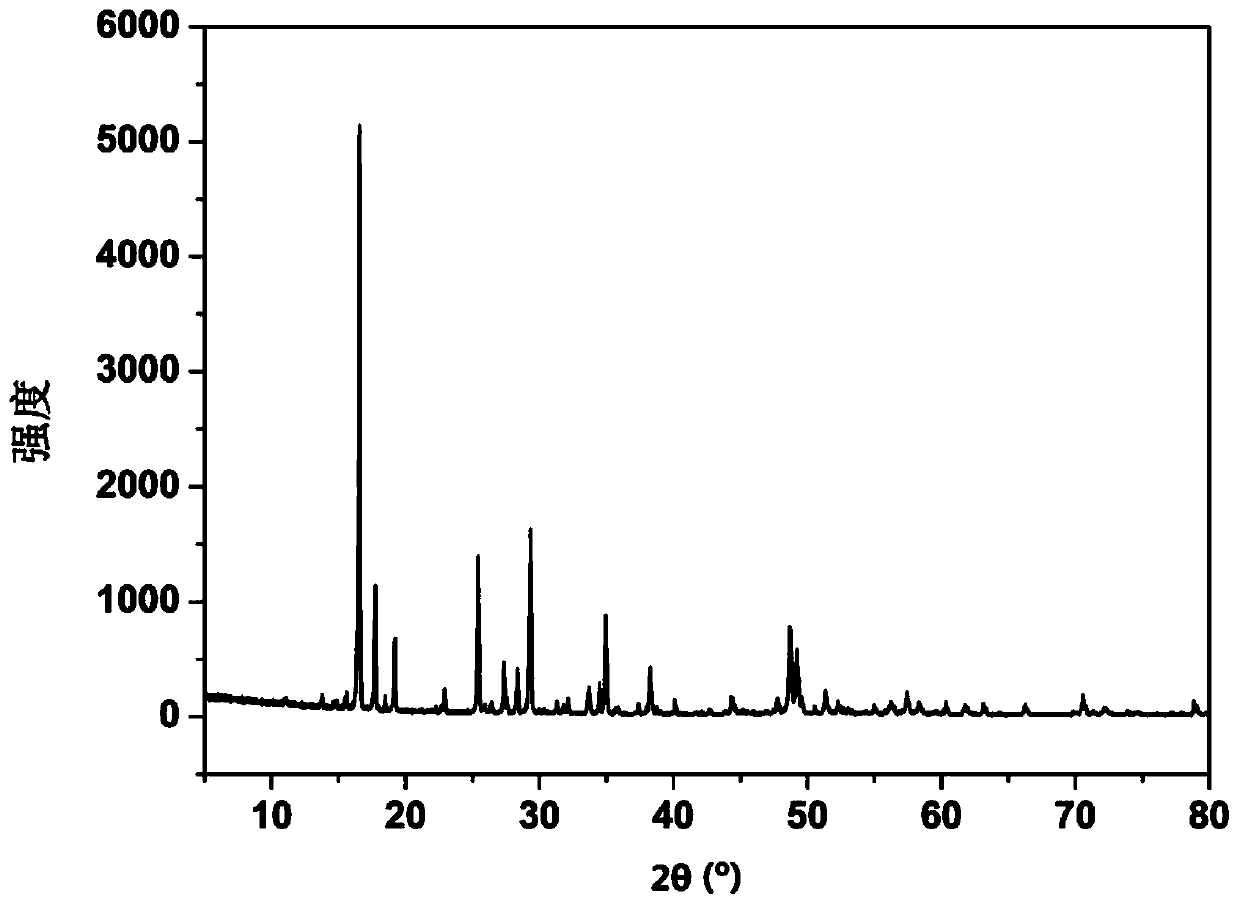
[0059] A certain amount of titanium tetrachloride was dissolved in the solution to detect the content of titanium and chlorine. The e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com