Preparation method of retegravir intermediate
A technology of remdesivir and intermediates, which is applied in the field of preparation of remdesivir intermediates, can solve the problem of poor selectivity of route 1, and achieve the effects of saving costs and improving chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
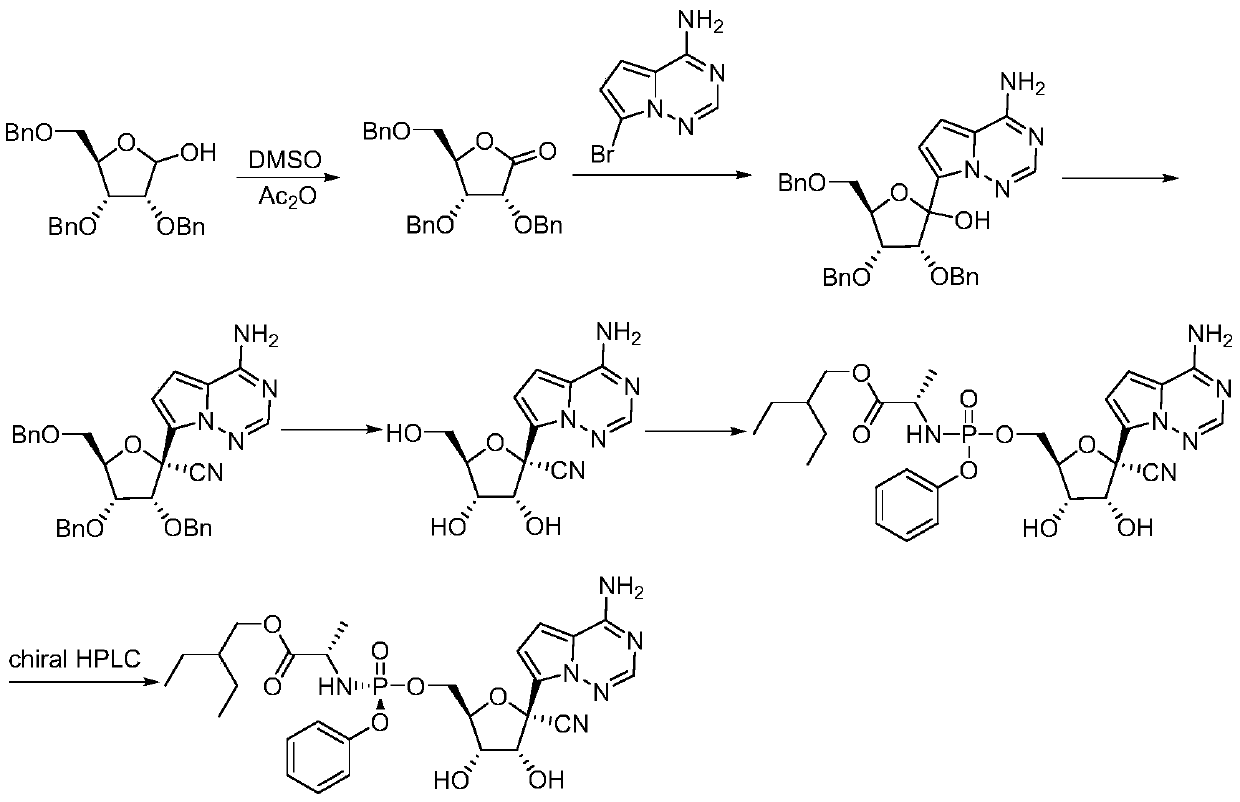
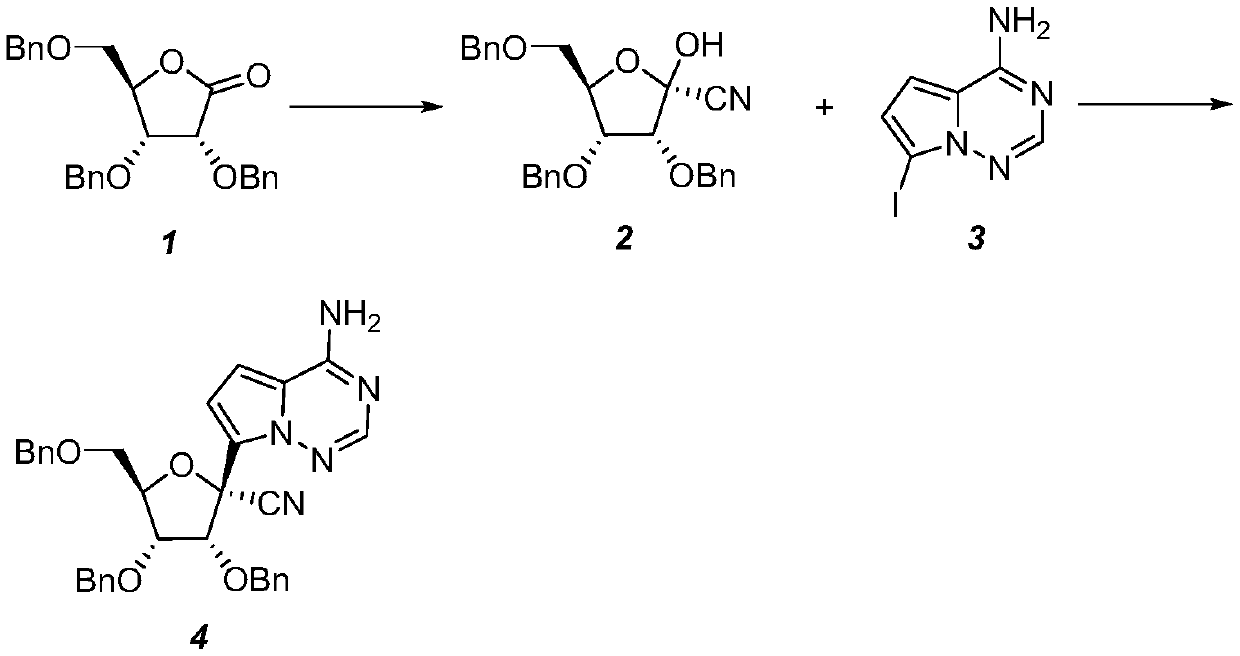
[0031] A (2R,3R,4R,5R)-2-(4-aminopyrrole[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy )-5-((benzyloxy) methyl) tetrahydrofuran-2-carbonitrile preparation method, concrete steps are:
[0032] 1. Synthesis of (2R,3R,4R,5R)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)-2-hydroxytetrahydrofuran-2-carbonitrile
[0033] Dissolve 2,3,5-tribenzyloxy-D-ribonic acid-1,4-lactone (41.9g, 0.1mol) in anhydrous dichloromethane (300mL), control the temperature at 0-10°C, drop Add titanium tetrachloride (5.5 mL, 0.05 mol), and then add trimethylsilyl cyanide (19.8 g, 0.2 mol) after the dropwise addition. After the dropwise addition, react for 1 hour, add 200 mL of saturated aqueous sodium bicarbonate solution to the reaction solution, stir and separate the liquids, dry the organic phase over anhydrous magnesium sulfate for 6 hours, remove the solvent under reduced pressure, and add ethyl acetate (50 mL) to the residue , petroleum ether (150mL), heated to 40°C and stirred to dissolve, then gradual...
Embodiment 2
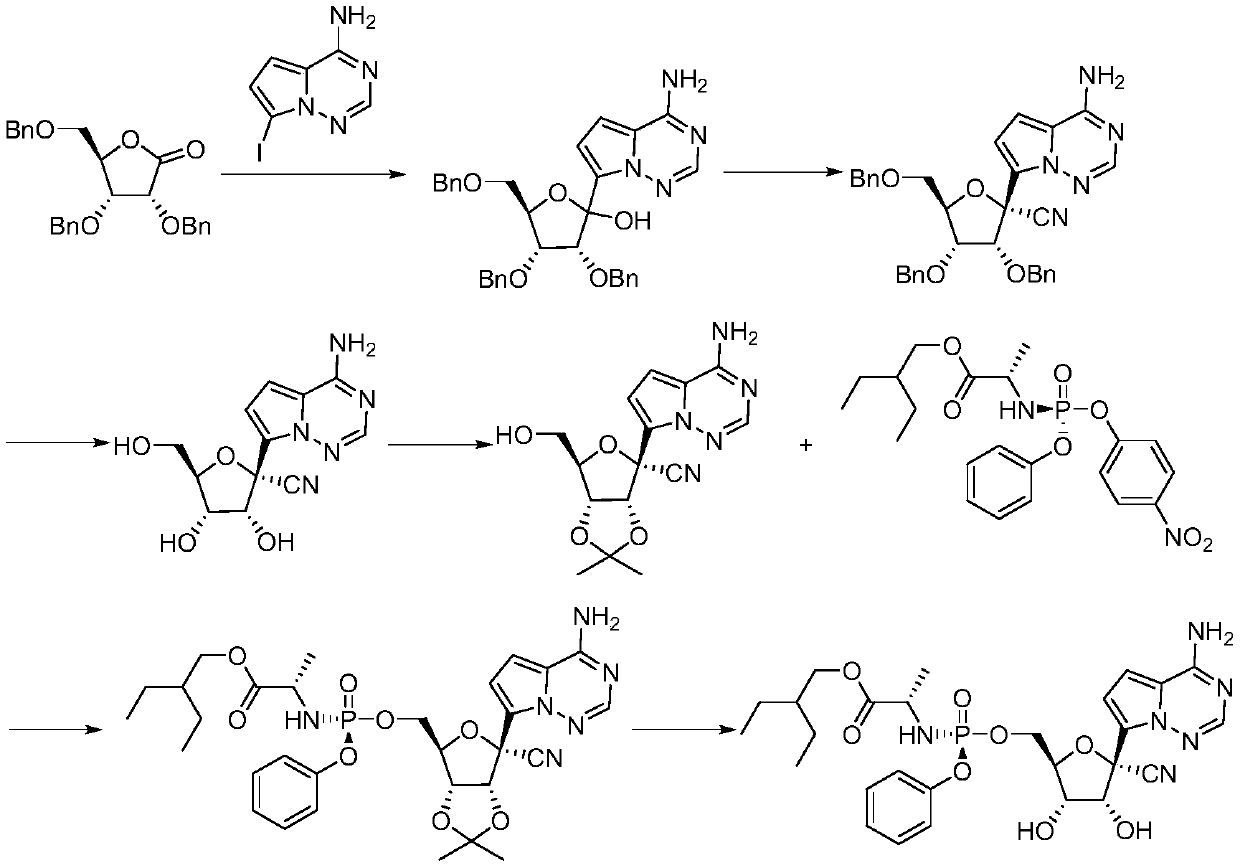
[0037] A (2R,3R,4R,5R)-2-(4-aminopyrrole[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy )-5-((benzyloxy) methyl) tetrahydrofuran-2-carbonitrile preparation method, concrete steps are:
[0038] 1. Synthesis of (2R,3R,4R,5R)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)-2-hydroxytetrahydrofuran-2-carbonitrile
[0039] Dissolve 2,3,5-tribenzyloxy-D-ribonic acid-1,4-lactone (41.9g, 0.1mol) in 1,2-dichloroethane (300mL) and control the temperature for 20-30 °C, add anhydrous aluminum trichloride (20 g, 0.15 mol), and then add tert-butylcyanodimethylsilane (56 g, 0.4 mol) after the dropwise addition. After the dropwise addition, react for 1 hour, add 200 mL of saturated aqueous sodium bicarbonate solution to the reaction solution, stir and separate the liquids, dry the organic phase over anhydrous magnesium sulfate for 6 hours, remove the solvent under reduced pressure, and add 4-methyl 2- Pentanone (50mL) and n-heptane (100mL) were heated to 45°C and stirred to dissolve, then gradually ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com