Rapid preparation method of MOF material
A fast, time-controlled technology, applied in chemical instruments and methods, other chemical processes, physical/chemical process catalysts, etc., can solve the problems of uncontrollable particle size, slow reaction process, incomplete crystal growth, etc., and achieve uniform particle size. , The effect of easy product and high industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
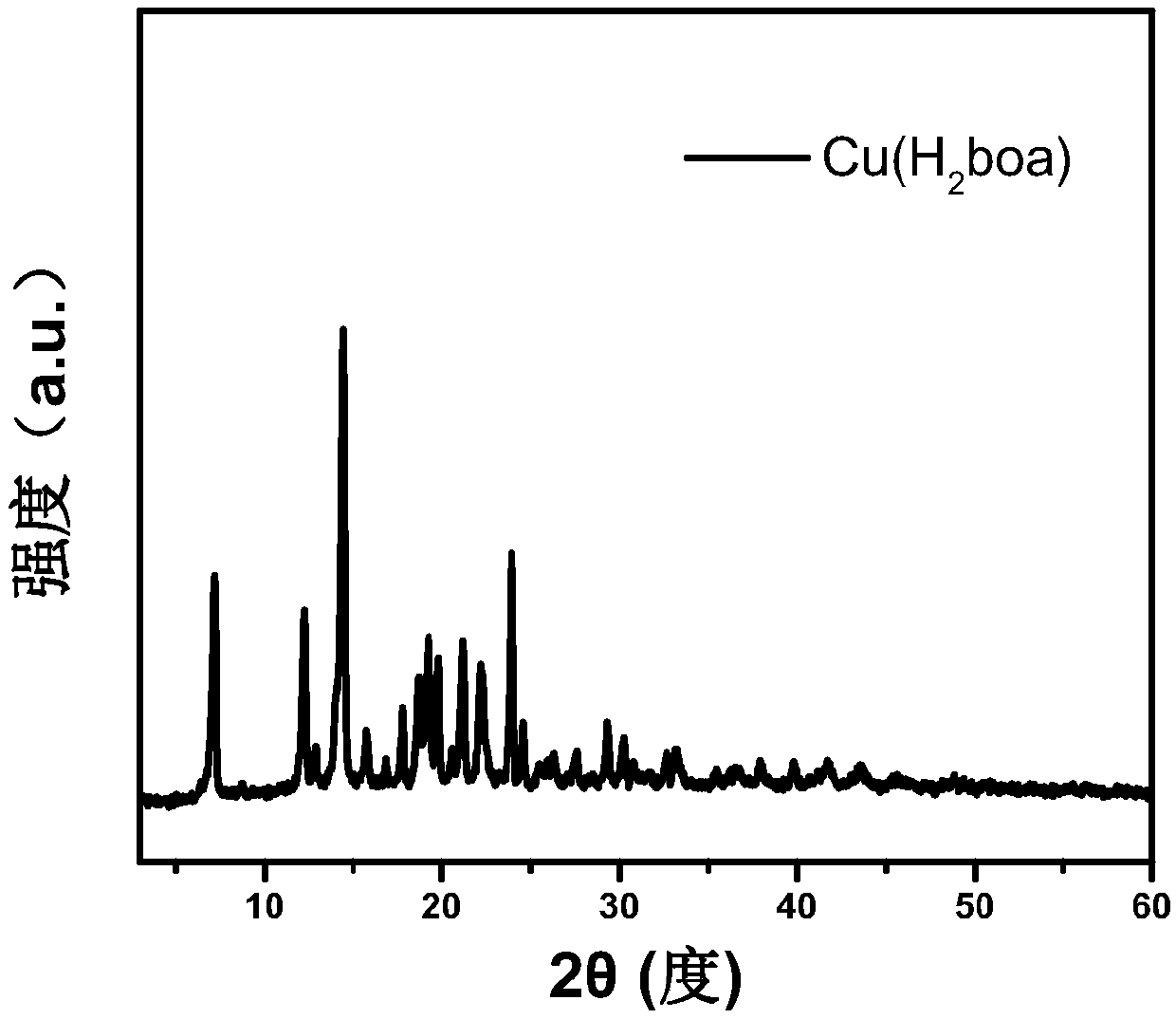
[0038] The MOF material Cu(H 2 oba):
[0039] In step (1), 100 mg of copper acetate was dissolved in 2 mL of water to obtain an aqueous solution of copper acetate, and 258 mg of 4,4'-diphenyl ether dicarboxylic acid was dissolved in 1.5 mL of N,N-dimethylformamide (DMF) to obtain an organic DMF solution of ligand;
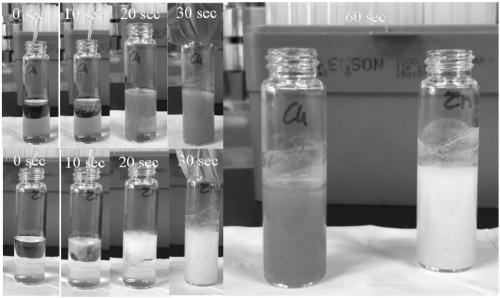
[0040] In step (2), the DMF solution of the organic ligand obtained in step (1) is dropped into the copper acetate aqueous solution at 30°C, and the time of dropping is controlled at about 50s to obtain a suspension of the crude product of the MOF material;
[0041] In step (3), the suspension of the MOF material crude product obtained in step (2) is centrifuged, and the precipitate obtained by centrifugation is cleaned with DMF and methanol respectively, which is the MOF material Cu(H 2 oba).
Embodiment 2
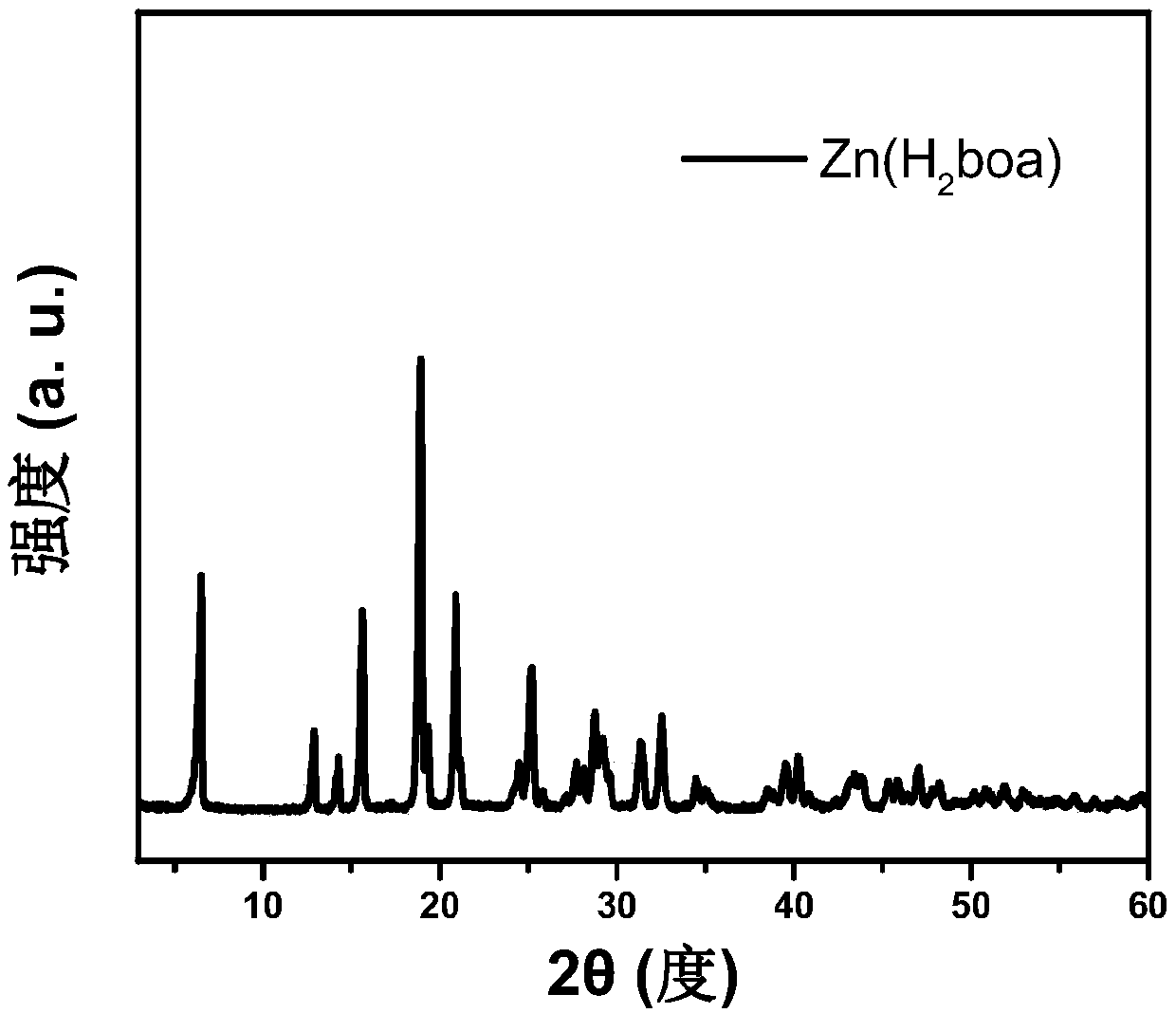
[0043] The MOF material Zn(H) was prepared by the following steps 2 oba):
[0044] In step (1), 87.6 mg of zinc acetate was dissolved in 2 mL of water to obtain an aqueous solution of zinc acetate, and 258 mg of 4,4'-diphenyl ether dicarboxylic acid was dissolved in 1.5 mL of N,N-dimethylformamide (DMF) to obtain DMF solution of organic ligand;
[0045] In step (2), the DMF solution of the organic ligand obtained in step (1) is dropped into the zinc acetate aqueous solution at 30° C., and the time of dropping is controlled at 20 to 30 s to obtain a suspension of the crude product of the MOF material;
[0046] In step (3), the suspension of the MOF material crude product obtained in step (2) is centrifuged, and the precipitate obtained by centrifugation is cleaned with DMF and methanol respectively, which is the MOF material Zn(H 2 oba).
Embodiment 3
[0048] The MOF material Cd(H) was prepared by the following steps 2 oba):
[0049] In step (1), 133mg of cadmium acetate was dissolved in 1mL of water to obtain an aqueous solution of cadmium acetate, and 258mg of 4,4'-diphenyl ether dicarboxylic acid was dissolved in 1.5mL of N,N-dimethylformamide (DMF) to obtain an organic DMF solution of ligand;
[0050] In step (2), the DMF solution of the organic ligand obtained in step (1) is dropped dropwise into the cadmium acetate aqueous solution at 35°C, and the time of dropping is controlled at 20-30s to obtain a suspension of the crude product of the MOF material ;
[0051] In step (3), the suspension of the MOF material crude product obtained in step (2) is centrifuged, and the precipitate obtained by centrifugation is cleaned with DMF and methanol respectively, which is the MOF material Cd(H 2 oba).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com