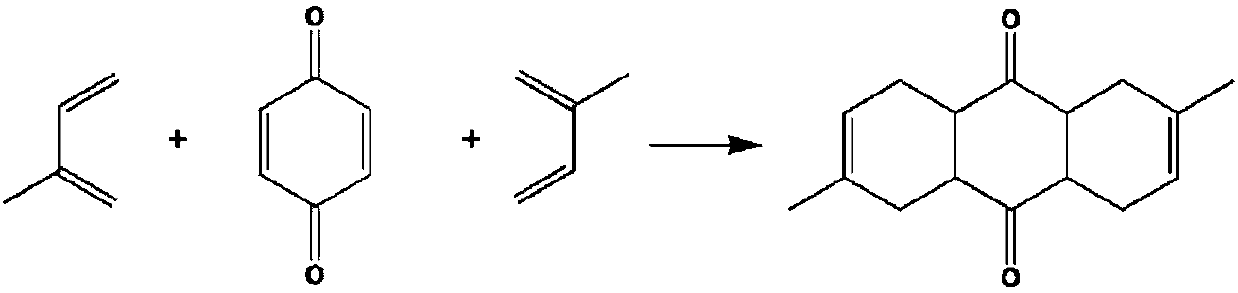
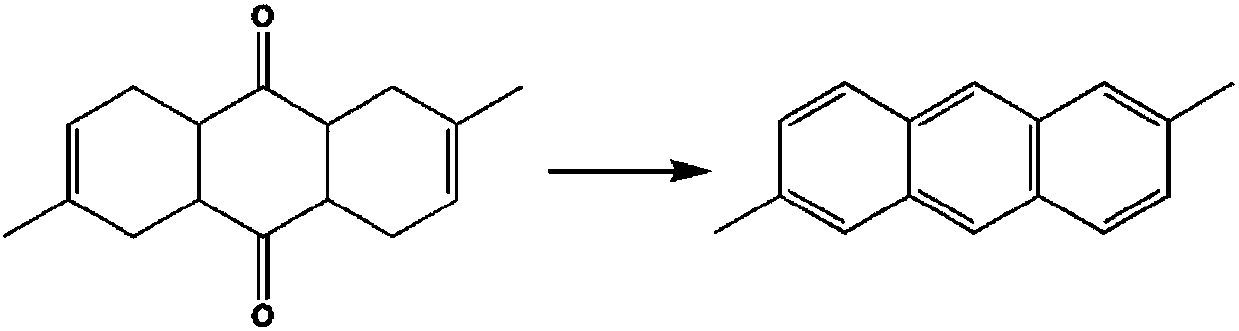
Method for preparing 2,6-dimethyl anthracene from isoprene and 1,4-benzoquinone
A technology of isoprene and dimethylanthracene, which is applied in the field of preparing 2,6-dimethylanthracene, can solve the problems of 2,6-dimethylanthracene, such as small yield, difficulty in obtaining, and high price, and achieves High product yield, short reaction route and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add a certain amount of isoprene and 1,4-benzoquinone into the hydrothermal reaction kettle, and react at different temperatures. After a certain period of time, take a sample and add about 1mL tetrahydrofuran to dilute, mix well and then centrifuge, and the product is separated by GC- MS qualitative, GC external standard quantitative analysis. The reaction results are shown in Table 1.
[0028] Table 1. Results of the reaction of isoprene and 1,4-benzoquinone under thermal conditions
[0029] .
[0030] The above results show that in the temperature range of 80°C-170°C, the reaction of isoprene / 1,4-benzoquinone for 20-60 hours can obtain dimethyl decahydroanthracene with a yield of 50%-95% Between, preferably at 80-130°C for 10-30 hours, the molar ratio of isoprene to 1,4-benzoquinone is greater than 2.5 to 1.
Embodiment 2
[0032] 3 milliliters of palladium nitrate solution with a concentration of 10 milligrams per milliliter was added to 6 grams of alumina carrier, impregnated for 5 hours, dried at 80° C. for 12 hours, and then calcined at 450° C. for 4 hours. Obtaining a mass content of 1wt% noble metal catalyst, denoted as 0.5Pd / Al 2 o 3 .
[0033] Catalysts 1Pd / Al with different loads were also prepared 2 o 3 , 5Pd / Al 2 o 3 , 10Pd / Al 2 o 3 , and catalysts with different supports 1Pd / C, 1Pd / SiO 2 .
Embodiment 3
[0035] The catalyst is prepared as in Example 2, except that the palladium nitrate solution is replaced by a chloroplatinic acid solution to prepare a platinum-based catalyst 1Pt / Al 2 o 3 ,1Pt / C, 1Pt / SiO 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com