A kind of near-infrared fluorescent dye and its preparation method and application
A fluorescent dye and near-infrared technology, applied in the field of medicine, can solve the problems of lack of lymphatic targeting, strong liver and kidney toxicity, and high price, and achieve the effect of simple, effective, high stability, and simple and easy method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
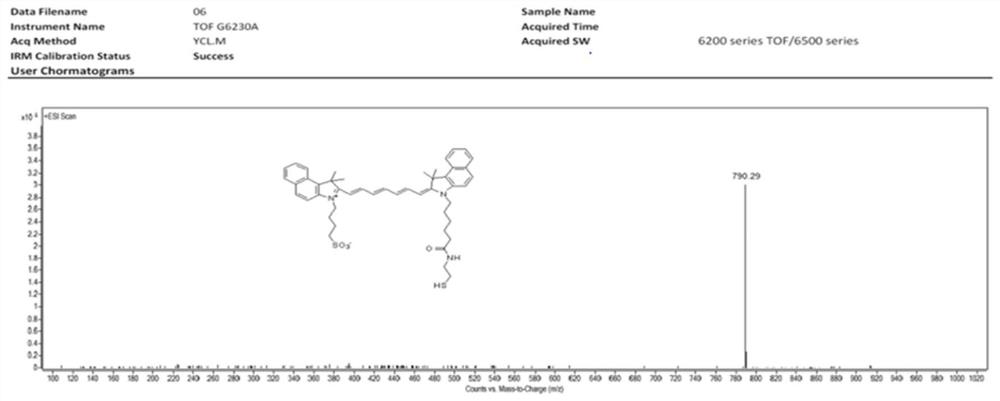
[0049] Example 1 Preparation of ICG-SH
[0050] The synthetic route is as follows:
[0051]
[0052] Dissolve 200mg of ICG-NHS in 25mL of dichloromethane, add 50μL of triethylamine, stir for 5 minutes, dissolve 100mg of trityl-protected aminothiol in 5mL of dichloromethane and slowly add to the reaction solution, and react at room temperature in the dark Stir for 6 hours. The reaction solution was concentrated to 5 mL and then poured into 50 mL of diethyl ether to obtain a green precipitate, which was dissolved in dichloromethane and placed on a silica gel column for separation to obtain 75 mg of trityl-protected ICG-SH.
[0053] Dissolve 75 mg of trityl-protected ICG-SH in 5 mL of trifluoroacetic acid, add 5 mL of triethylsilane, and react in the dark at room temperature for 4 hours. After the reaction solution is concentrated, it is poured into 25 mL of ether to obtain a green precipitate, and the precipitate is dissolved in In methanol, HPLC separation and purificati...
Embodiment 2
[0056] The preparation method of embodiment 2 ICG-SH-GNPs
[0057] Slowly drop 1ml of DMSO solution of ICG-SH (protected from light) into 1ml of acrylamide solution of nano-gold with a particle size of 20 nm, the molar ratio of ICG-SH to nano-gold is 1:4, stir for 3 hours, and dialyze at 3500Ka bag, and dialyzed for 48 hours to obtain pure ICG-SH-GNPs solution, which was stored in a 4°C refrigerator for later use. The electron microscope image of ICG-SH-GNPs is shown in image 3 shown.
Embodiment 3
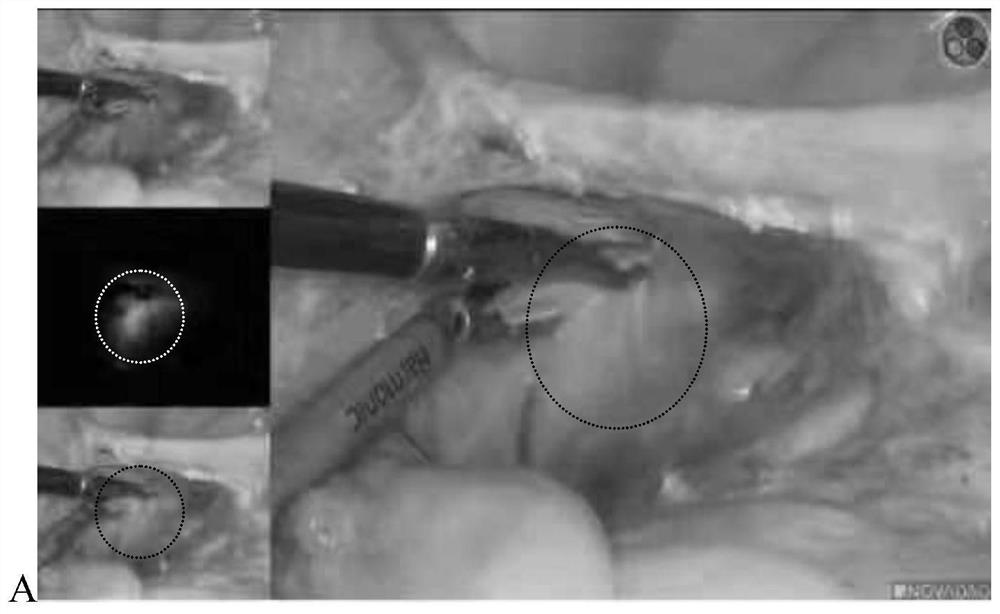
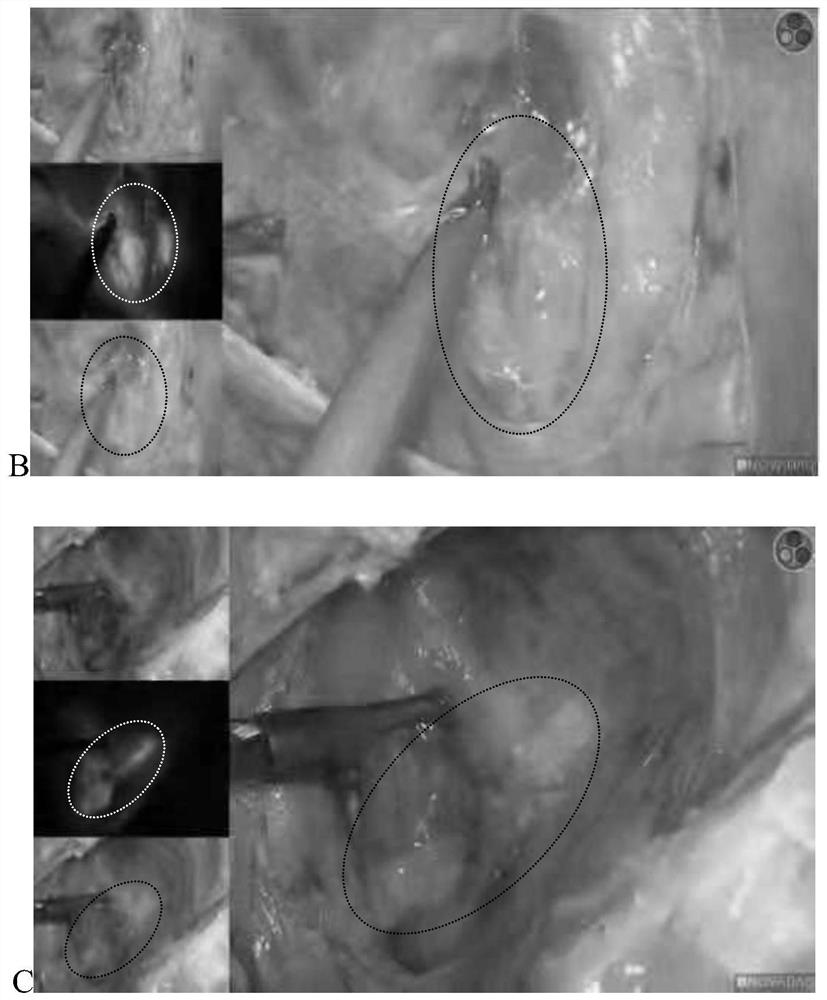
[0059] Experimental method: patients with endometrial cancer were slowly injected with 0.5ml of ICG-SH (prepared in Example 1) at 0, 3, and 9 o'clock in the cervical stroma. Like the case, the result is like figure 2 shown.
[0060] figure 2 It is the imaging results of lymph nodes at different time points after local injection of ICG-SH in the cervix, where A is the imaging results of lymph nodes 10 minutes after injection, B is the imaging results of lymph nodes 30 minutes after injection, and C is the imaging results of lymph nodes 60 minutes after injection. like result.
[0061] according to figure 2 It can be seen that ICG-SH can stain lymph nodes well after infrared irradiation, and the staining time can last for more than 60 minutes. figure 2 As can be seen in Figure C, the lymph nodes can still be clearly traced after 60 minutes of injection, and have not spread to the entire cervical tissue, indicating that ICG-SH has higher stability, less diffusion and long...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com