High-capacity imidazole ionic liquid as well as preparation method and application thereof
A technology of imidazole ionic liquid and ionic liquid, which is applied in the application field of new imidazole ionic liquid and lithium-ion battery electrolyte, and can solve the problems that pure ionic liquid electrolyte cannot achieve electrochemical effect, poor cycle stability, and poor rate performance. , to achieve good cycle performance and electrochemical stability, high stability, and wide electrochemical window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] In addition, this embodiment also provides a preparation method of imidazolium ionic liquid, comprising the following steps:
[0057] In step S110, according to the molar ratio of 2-methylimidazole and brominated alkyl ether being 1:1 to 1:1.1, 2-methylimidazole and brominated alkyl ether are dissolved in a solvent at 100 to 150 Reaction at ℃ for 24-48 hours; after the reaction, extract with a mixed solution of absolute ethanol and absolute ether for 2-3 times, and then add triethylamine in an equimolar amount to 2-methylimidazole in the organic phase, at 100-130 Reaction at ℃ for 16 to 24 hours to obtain 1-alkoxyalkyl-2-methylimidazole of the following structural formula:
[0058]
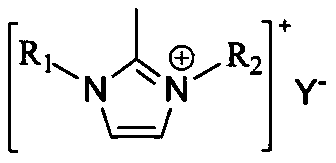
[0059] Among them, R 1 for CH 2 (CH 2 ) a O(CH 2 ) b CH 3 , a is 0 or 1, b is 0 or 1;
[0060] The obtained product contains 1-alkoxyalkyl-2-methylimidazole, and the white salt impurities generated by the reaction are removed by suction filtration, the filtrate is collected, and...
Embodiment 1
[0085] Take 0.1mol 2-methylimidazole, 0.105mol 2-bromoethyl methyl ether and 20mL of absolute ethanol, mix and dissolve, transfer to a 250ml reaction kettle, and react at 140°C for 48 hours; then use 10ml of absolute ethanol and The mixed solution of anhydrous ether (1:4, v / v) extracted the brown solution generated by the reaction three times, kept the organic phase solution, then mixed with 0.1mol triethylamine and transferred to a 250mL reactor, and reacted at 130°C for 16 hours; remove the white salts of the reaction product by suction filtration, collect the filtrate and carry out vacuum distillation, and collect the fractions to obtain 1-methoxyethyl-2-methylimidazole.
[0086]
[0087] Among them, R 1 for CH 2 (CH 2 ) a O(CH 2 ) b CH 3 , a is 1, b is 0;
[0088] Under nitrogen protection gas atmosphere, dissolve 0.083mol 1-methoxyethyl-2-methylimidazole in 15mL dichloromethane, then mix and dissolve with 0.087mol methyl chloroacetate, and then put the above mi...
Embodiment 2
[0104] Take 0.1mol 2-methylimidazole, 0.105mol 2-bromoethyl ethyl ether and 20mL of absolute ethanol, mix and dissolve, transfer to a 250ml reaction kettle, and react at 140°C for 48 hours; then use 10ml of absolute ethanol and anhydrous A mixed solution of water and ether (1:4, v / v) washed the brown solution generated by the reaction three times, then mixed with 0.1mol triethylamine and transferred to a 250mL reactor, and reacted at 130°C for 16 hours; the reaction solution was removed by suction filtration. The product is a white salt substance, the filtrate is collected and distilled under reduced pressure, and the fraction is collected to obtain 1-ethoxyethyl-2-methylimidazole.
[0105] Under nitrogen protection gas atmosphere, dissolve 0.083mol 1-ethoxyethyl-2-methylimidazole in 15mL dichloromethane, then mix and dissolve with 0.087mol methyl chloroacetate, then react the above mixture at 60°C After 48 hours, the reaction product was obtained; the product was distilled un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com