Imidazole ionic liquid as well as preparation method and application thereof
A technology of imidazolium ionic liquid and ionic liquid, applied in electrochemical generators, electrical components, organic electrolytes, etc., can solve the problems of insufficient electrochemical performance of ionic liquids, achieve excellent electrochemical performance, improve electrical conductivity, and facilitate reaction The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] In addition, this implementation also provides a kind of preparation method of imidazole ionic liquid, comprises the steps:
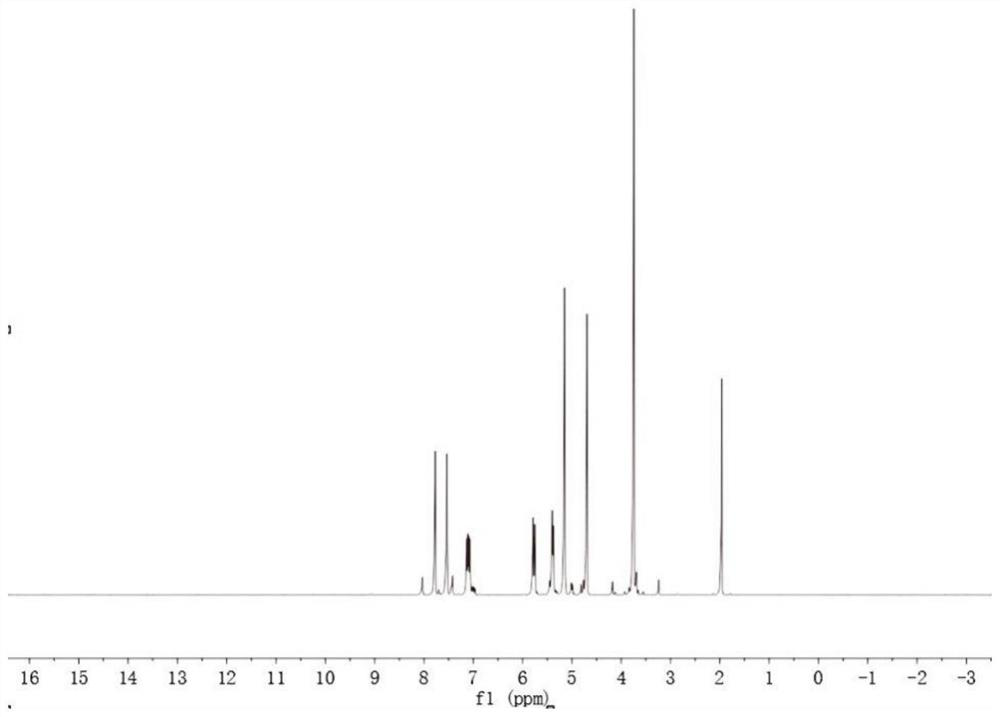
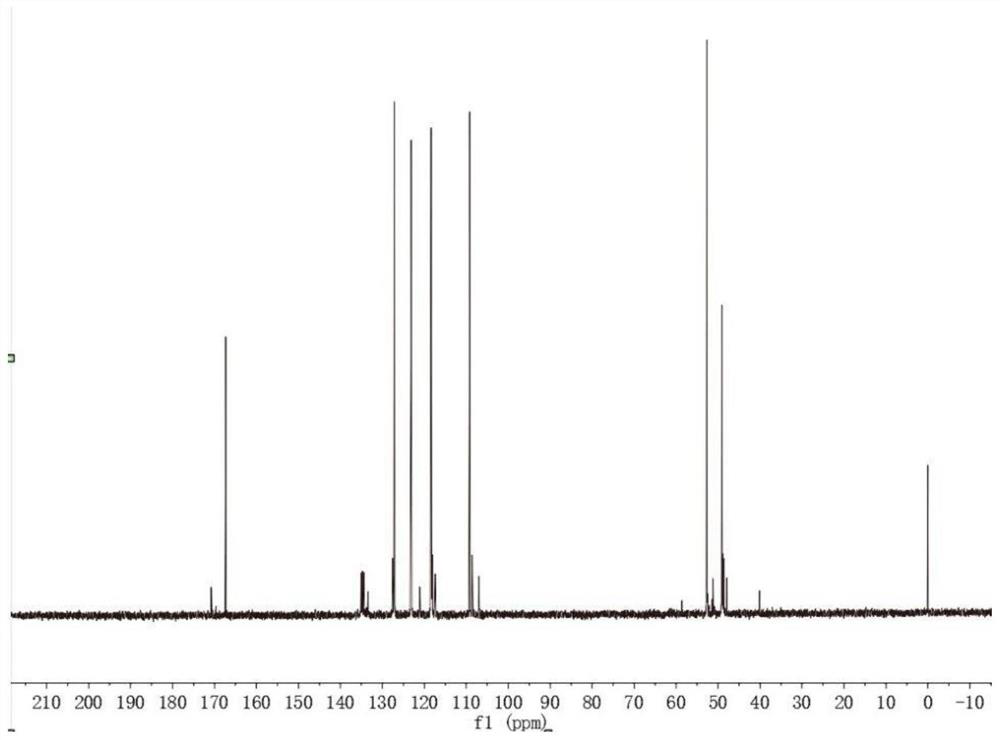
[0053] Under the protective gas nitrogen atmosphere, dry the reactant before the reaction to remove impurities. First, put 1-vinylimidazole in a drying oven at 60°C for 12 hours, and then dissolve 1-vinylimidazole and chloroalkyl ester in the solvent. , do multiple sets of experiments, the ratio is 1:1.1~1:1.3, and then stir and reflux reaction at 65℃~80℃ for 30~48 hours to obtain the reactant, stop the reaction, rotary evaporate, and the reacted The reaction product was vacuum-dried at 50°C-65°C for 20-30 hours to obtain a yellow transparent viscous liquid and 1-vinyl-3-ester imidazole halide with the following structural formula. Obtain the following structural formula 1-vinyl-3-ester imidazole halide:
[0054]
[0055] R is CH 2 (CH 2 ) a COO(CH 2 ) b CH 3 , a is 0 or 1, b is 0 or 1; Y - for halogen.
[0056] In other preferred em...
Embodiment 1
[0074] Under the protection of nitrogen atmosphere, 0.1 mol of 1-vinylimidazole, 0.1 mol of methyl chloroacetate and 30 ml of acetonitrile were added to a 100 ml three-necked flask, and then stirred and refluxed at 70 ° C for 48 hours to obtain the reactant. After the reaction was stopped, spin Unreacted acetonitrile and methyl chloroacetate were removed by evaporation, and the reacted reaction product was vacuum-dried at 60°C for 24 hours to obtain a yellow transparent liquid: 1-vinyl-3-acetate methyl imidazole chloride.
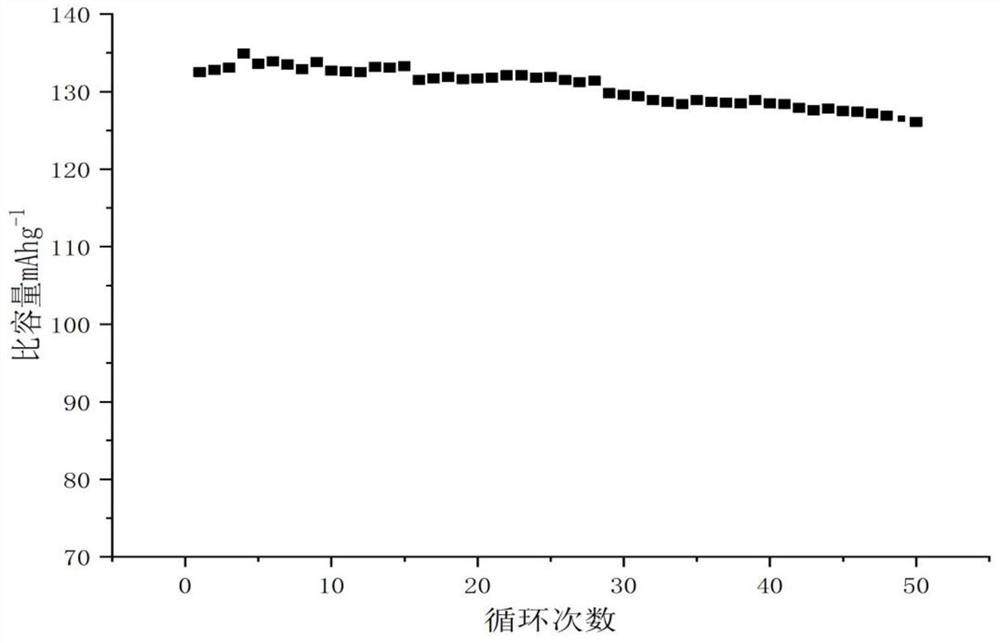
[0075] In the experiment, the inventor found that after the product (1-vinyl-3-acetate imidazole chloride) was left standing at room temperature for 3 hours, the liquid began to gradually transform into a solid, and the color changed from obvious yellow to off-white solid , if this solid is directly used for the second step of anion exchange, it will be completely insoluble after stirring for 24 hours at room temperature. So need to improve. In the improve...
Embodiment 2
[0087] Under the protection of nitrogen atmosphere, 0.1mol 1-vinylimidazole, 0.11mol methyl chloroacetate and 30ml acetonitrile were added to a 100ml three-necked flask, and then stirred and refluxed at 70°C for 48 hours to obtain the reactant. After the reaction was stopped, spin Unreacted acetonitrile and methyl chloroacetate were removed by evaporation, and the reacted reaction product was vacuum-dried at 60°C for 24 hours to obtain a yellow transparent liquid: 1-vinyl-3-acetate methyl imidazole chloride. In the experiment, it was found that after the product was left standing at room temperature for 3 hours, the liquid began to gradually transform into a solid, and the color changed from obvious yellow to off-white solid. If this solid is directly used for the second step of anion exchange, at room temperature, Complete insolubility was apparent upon stirring for 24 hours. So need to improve. In the improvement measures, the problem of product purification was considered....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com