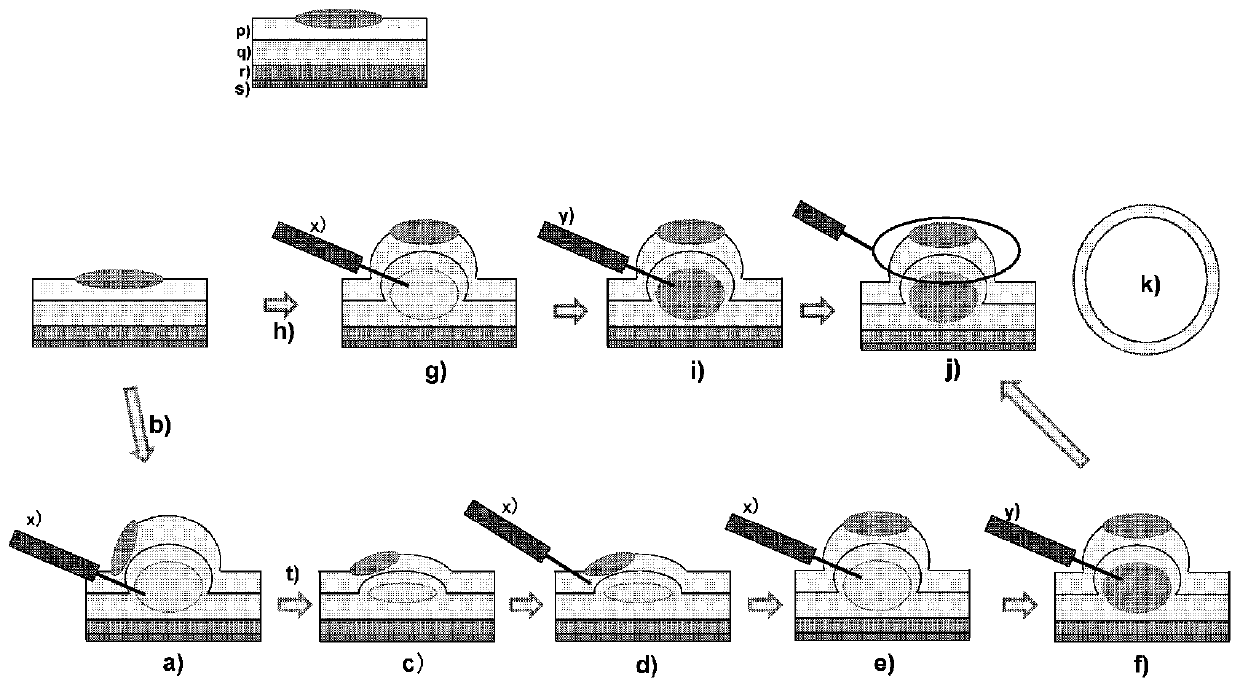
Two-drug type local injection solution for submucosal injection
A local injection, mucosal technology, used in liquid delivery, aerosol delivery, emulsion delivery, etc., can solve the problem of not developing a local injection solution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0067] The present invention will be described in detail below in conjunction with examples and comparative examples.
[0068] In the examples, the following starting materials were used.
[0069] Sodium Alginate:
[0070] Product name Sodium alginate produced by Nacalai Tesque Co., Ltd.
[0071] Gellan Gum:
[0072] Product name KELCOGEL (CG-LA) produced by Sanjing Co., Ltd.
[0073] Carrageenan:
[0074] Product name GENUVISCO produced by Sanjing Co., Ltd. (CF02)
[0075] Sodium hyaluronate:
[0076] Product name MucoUp produced by Seikagaku Industry Co., Ltd.
preparation example 1
[0078] The following polysaccharide aqueous solutions and aqueous salt solutions were prepared as local injection solutions.
[0079] -Polysaccharide aqueous solution:
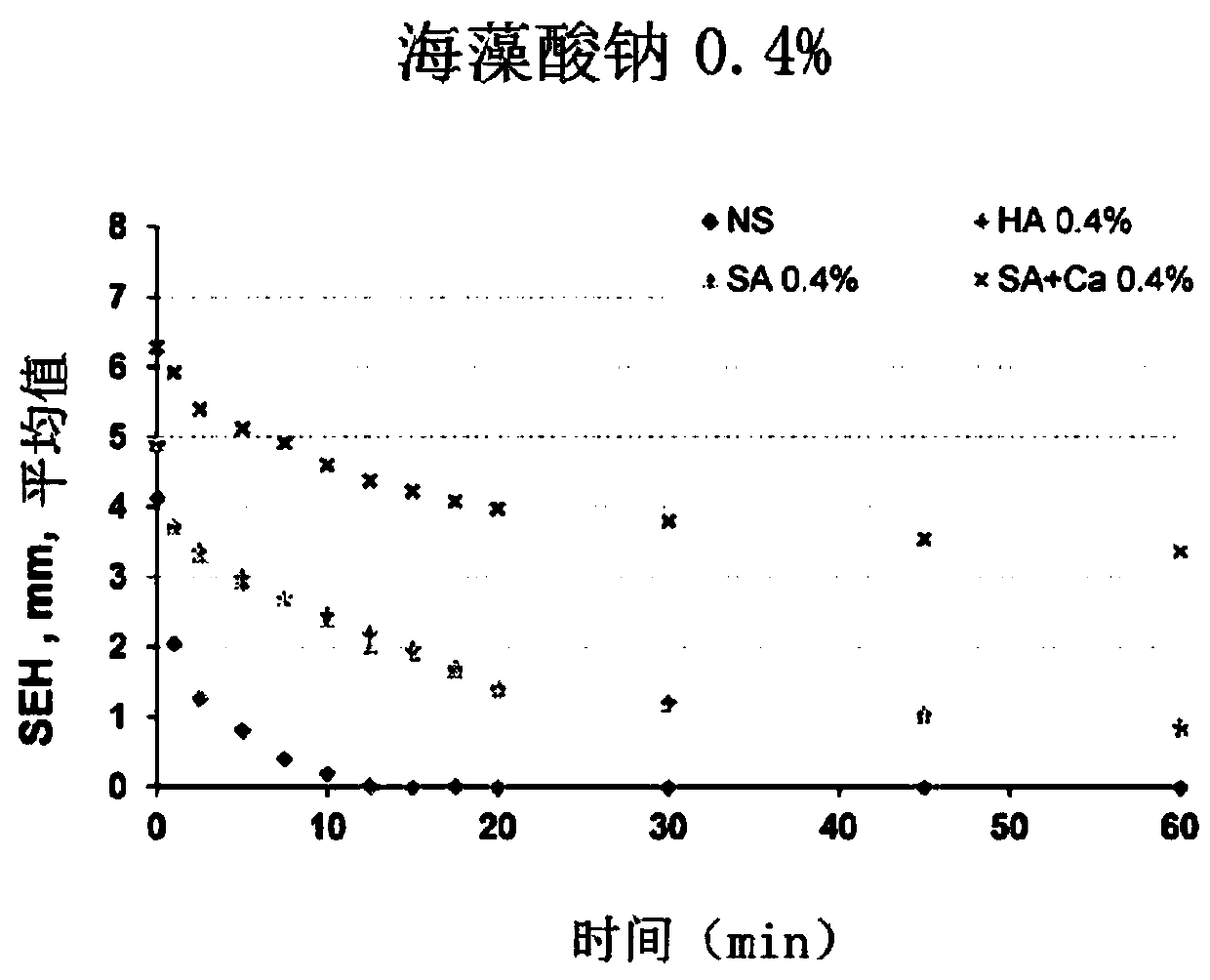
[0080] An aqueous solution of gellan gum (G-LA, 0.4%), hyaluronic acid (HA, 0.4%), sodium alginate (SA, 0.4%) or carrageenan (Agar, 1.0%) was prepared according to conventional methods.
[0081] -Aqueous salt solution:
[0082] Prepare 0.9% NaCl aqueous solution or 2.0% CaCl according to conventional methods 2 Aqueous solution.
[0083] These aqueous polysaccharide solutions and aqueous salt solutions were subjected to the following tests.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com