Application of atrial natriuretic peptide in preparation of medicine for treating inflammatory bowel diseases
An atrial natriuretic peptide, inflammatory bowel disease technology, applied in the field of biomedicine, can solve problems such as inability to use IBD, metabolic disorders, maintenance therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Therapeutic Effect on Mice Ulcerative Colitis
[0020] Material:
[0021] A. Mice: C57BL / 6J breed mice (purchased from Beijing Weitong Lihua Company), bred to 20-22g under SPF environment, about 8 weeks old.
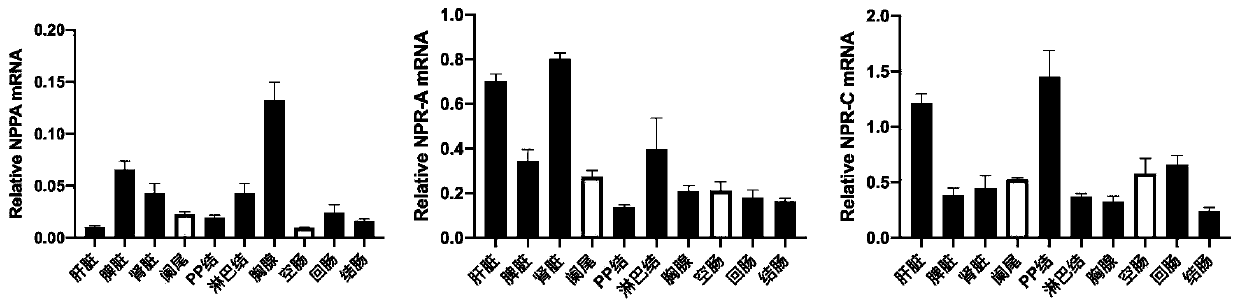
[0022] B. Human atrial natriuretic peptide ANP (purchased from Tocris Bioscience, UK), amino acid sequence Ser-Leu-Arg-Arg-Ser-Ser-Cys-Phe-Gly-Gly-Arg-Met-Asp-Arg-Ile-Gly - Ala-Gln-Ser-Gly-Leu-Gly-Cys-Asn-Ser-Phe-Arg-Tyr, molecular weight 3080.46 Da; purity 95.8%.
[0023] Method and Results:
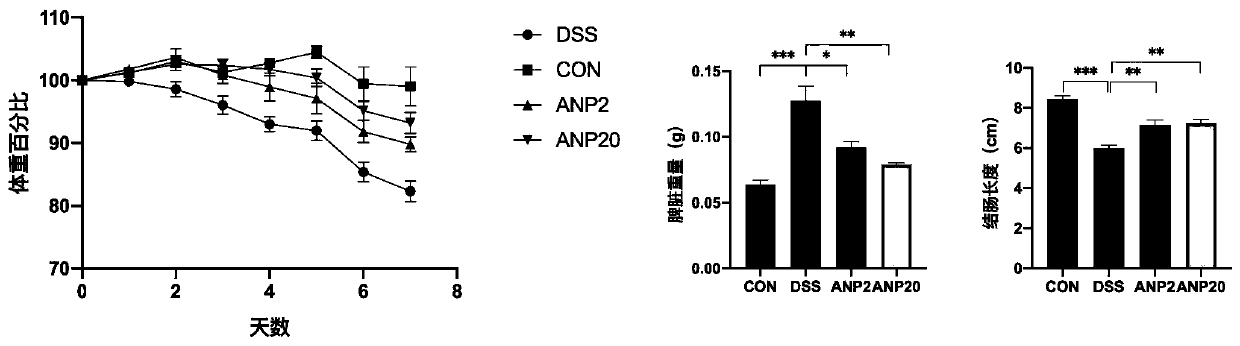
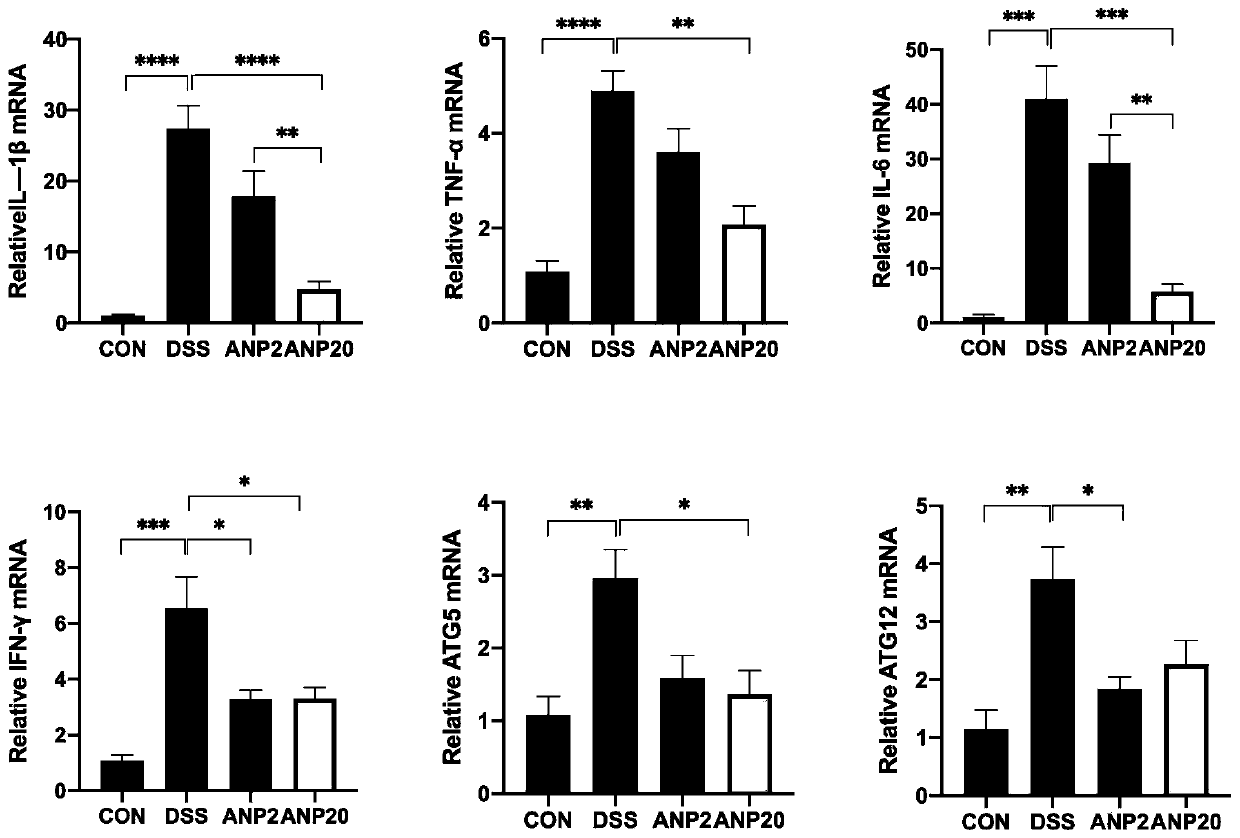
[0024] 1. Construction of dextran sodium sulfate (DSS)-induced colitis mouse model and the intervention of ANP recombinant protein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com