Steroid derivative and application thereof
A technology of derivatives and steroids, applied in the field of organic compounds, can solve the problems of restricting the application of glucocorticoids, and achieve the effect of relieving colonic inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 (preparation of compound)
[0019] The steroidal derivative described in this example consists of the following steps:
[0020] step one:
[0021] Dissolve 2.1g of sodium hydroxide in 18mL of water, add 2.88g of bromine dropwise in an ice bath, and when the bromine is completely dissolved, add the mixture to 12mL of dioxane to obtain a mixture; take the mixture and add 1.44g of pregnenolone acetic acid A solution of ester (1) (a mixed solution of 56 mL of dioxane and 16 mL of water), was stirred overnight, then sodium sulfite was added, heated to reflux until the solid was completely dissolved, and a white solid was precipitated after acidification with hydrochloric acid, and the solid was washed with water until the solution was colorless.
[0022] The chemical reaction formula of above-mentioned step 1 is as follows:
[0023]
[0024] The obtained white solid was identified by nuclear magnetic resonance spectrum, and the identification result was: 1H...
Embodiment 2
[0030] Embodiment 2 (cell experiment research)
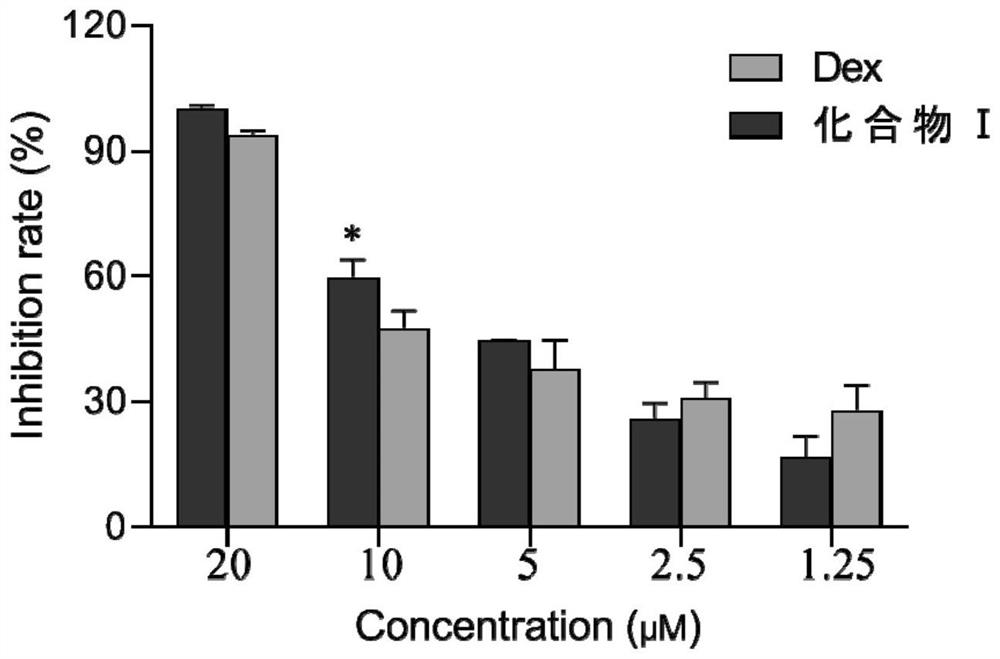
[0031] 1. The purpose and principle of the experiment
[0032] Experimental purpose: The NO inhibition test was used to detect the inhibitory effect of a series of steroids synthesized on the production of NO by LPS-stimulated macrophages.
[0033] Experimental principle: NO is easily oxidized in vivo or in aqueous solution to generate NO 2 - , under acidic conditions, NO 2 - Diazonium reaction with diazonium salt sulfonamide, and generate diazo compound, the latter further reacts with naphthyl vinyl diamine, the product concentration of this reaction is the same as that of NO 2 - The concentration has a linear relationship, and there is a maximum absorption peak at 540nm-560nm.
[0034] 2. Basic information of reagents
[0035] Reagent name brand NaNO 2
Macklin N-1-Naphthylethylenediamine hydrochloride Macklin 4-aminobenzenesulfonic acid Macklin RPMI-1640 Basal Medium Gibco ...
Embodiment 3
[0061] Embodiment 3 (animal experiment research)
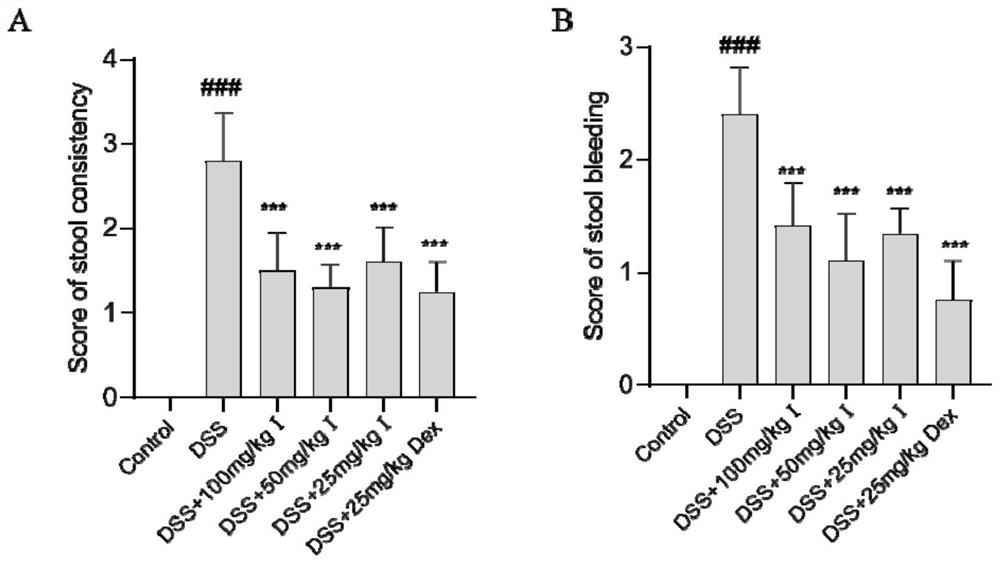
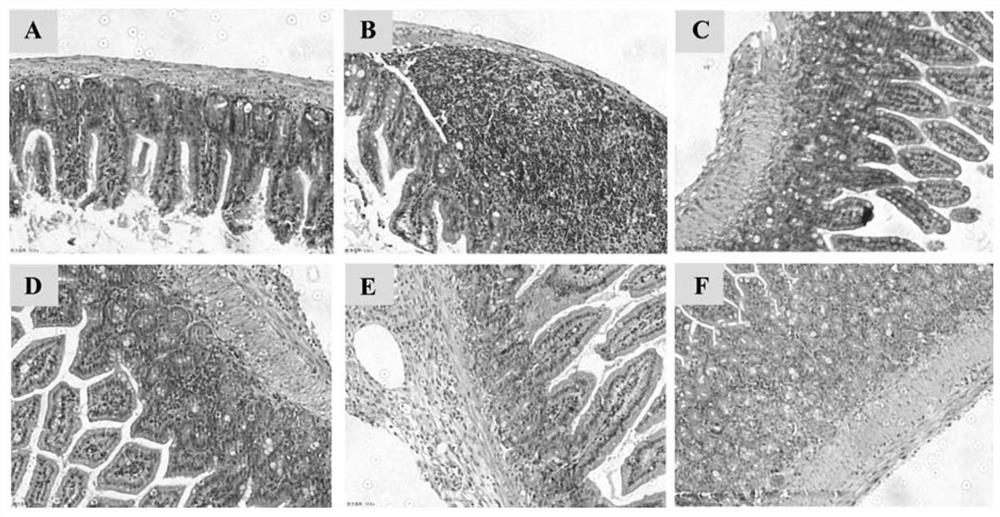
[0062] 1. Animal model
[0063] In the development of new drugs for inflammatory bowel disease, the dextran sodium sulfate (Dextran Sulfate Sodium Salt, DSS)-induced colitis (ulcerative colitis, UC) model is most widely used. By giving mice free to drink different concentrations of DSS aqueous solution, according to the time and cycle of medication, two colitis models, acute and chronic, can be established. The symptoms of this model are very similar to those of human UC, mainly manifested as diarrhea, mucoid stool, fecal occult blood, gross bloody stool, weight loss, decreased activity, and poor coat color. The main manifestations of the chronic phase colitis model are obvious shortening of the colon, thickening of the mucosa, enlargement of the lymph nodes, loss of goblet cells and loss of crypts, and a small number of animals have adenomatous polyps and tumor-like changes. The acute stage colitis model mainly manifests co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com