Application of lactobacillus plantarum L168 in assisting sorafenib in treating liver cancer
A technology of Lactobacillus plantarum and L168, which is applied in the field of Lactobacillus plantarum L168 assisted sorafenib in the treatment of liver cancer, can solve the problems of increased GSH, attenuated ferroptosis, and increased NRF2, so as to relieve colon inflammation, promote ferroptosis, and improve intestinal The effect of the structure of the intestinal flora
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation method of embodiment 1 Lactobacillus plantarum L168 bacterial liquid
[0023] Prepare MRS medium: weigh casein peptone 10.0g, beef extract 10.0g, yeast extract 5.0g, glucose 5.0g, sodium acetate 5.0g, diamine citrate 2.0g, Tween 80 1.0g, dipotassium hydrogen phosphate 2.0 g, magnesium sulfate heptahydrate 0.2 g, manganese sulfate heptahydrate 0.05 g, calcium carbonate 20.0 g, distilled water 1.0 liter, pH 6.8. The bacteria were seeded in MRS liquid medium at a ratio of 1:500, cultured in an anaerobic incubator at 37°C, and the bacterial concentration was determined according to bacterial absorbance (OD600) and bacterial plate count. Cultivate to the end of the logarithmic phase, remove the bacterial liquid, centrifuge at 12000rpm / min for 15-20min, wash with PBS for 2-3 times, and adjust the concentration to 1×10 with PBS 9 CFU / ml was used for gavage in mice.
Embodiment 2
[0024] Example 2 Lactobacillus plantarum L168 can promote the killing effect of sorafenib on liver cancer
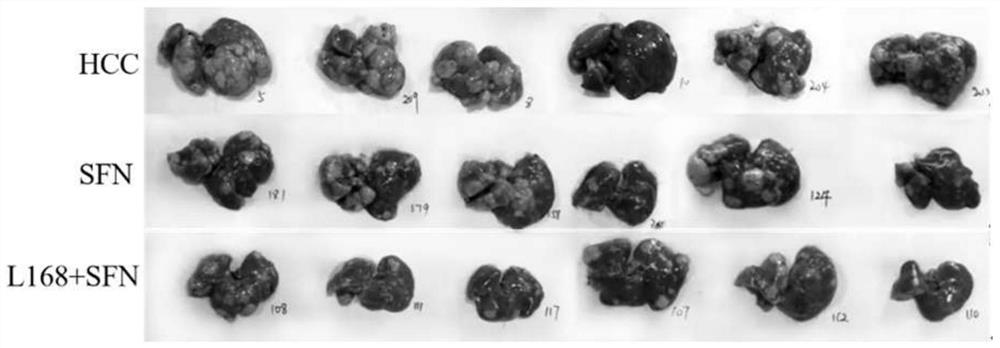
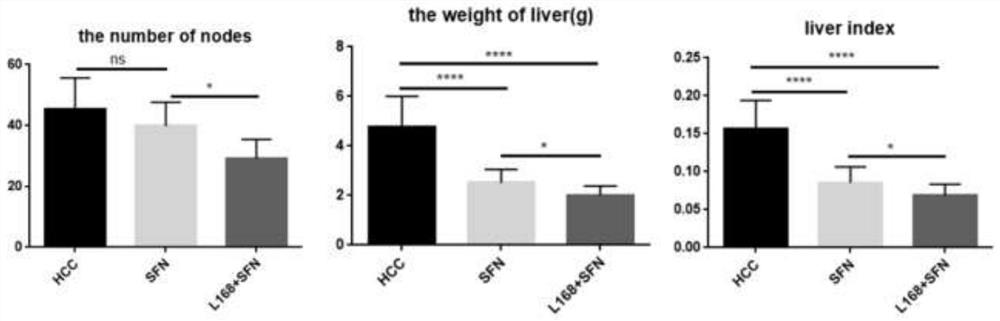
[0025] A mouse model of primary liver cancer was constructed by intraperitoneal injection of DEN and CCL4 into C57BL / 6 mice. During the modeling process, the mice were gavaged with the bacterial solution of the present invention and sorafenib, and the animal body weight, tumor weight and volume were analyzed. The chemical HCC models were randomly divided into 3 groups: liver cancer model control group, sorafenib single treatment group and sorafenib strain combination treatment group of the present invention, each group containing 10 mice.
[0026] Liver cancer chemical model mice were injected intraperitoneally with DEN reagent, 25 mg / kg, according to their body weight on the 14th day of birth. From the fourth week of birth, CCL4 reagent with a concentration of 20% was intraperitoneally injected once a week, 5ul / g, for 16 weeks. During the modeling process, the mice in t...
Embodiment 3
[0029] Example 3 Lactobacillus plantarum L168 can alleviate the side effects of intestinal inflammation caused by sorafenib treatment
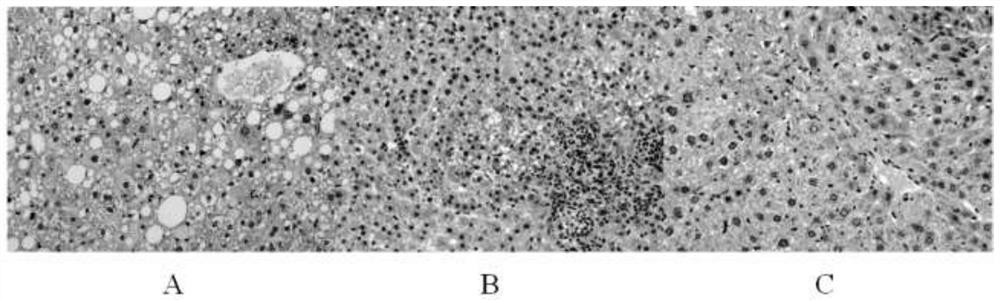
[0030] The colorectum of the mice in the liver cancer chemical model group in Example 2 was collected, and the intestinal lengths of the mice in the liver cancer model control group, the Sorafenib single treatment group and the Sorafenib combined treatment group with the strain of the present invention were measured, as shown in the following figure: Figure 4 As shown, compared with the sorafenib treatment group, the intestinal tract of the mice in the combined treatment group showed an increasing trend, and the H&E staining results were as follows Figure 5 It can be clearly seen from the slices that, compared with the liver cancer model control group and the sorafenib single treatment group, the intestinal inflammation of the mice has a tendency to be alleviated after the strain of the present invention is administered orally, Image 6 The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com