Preparation method of 1,1'-bis (tert-butylphosphine) ferrocene palladium chloride
A technology of tert-butylphosphine and di-tert-butylphosphine, which is applied in the field of preparation of 1,1'-ferrocenepalladium chloride, can solve the problems of unfavorable environment and safety, low catalyst quality, long reaction time, etc. , to achieve broad market prospects and benefits, low cost, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
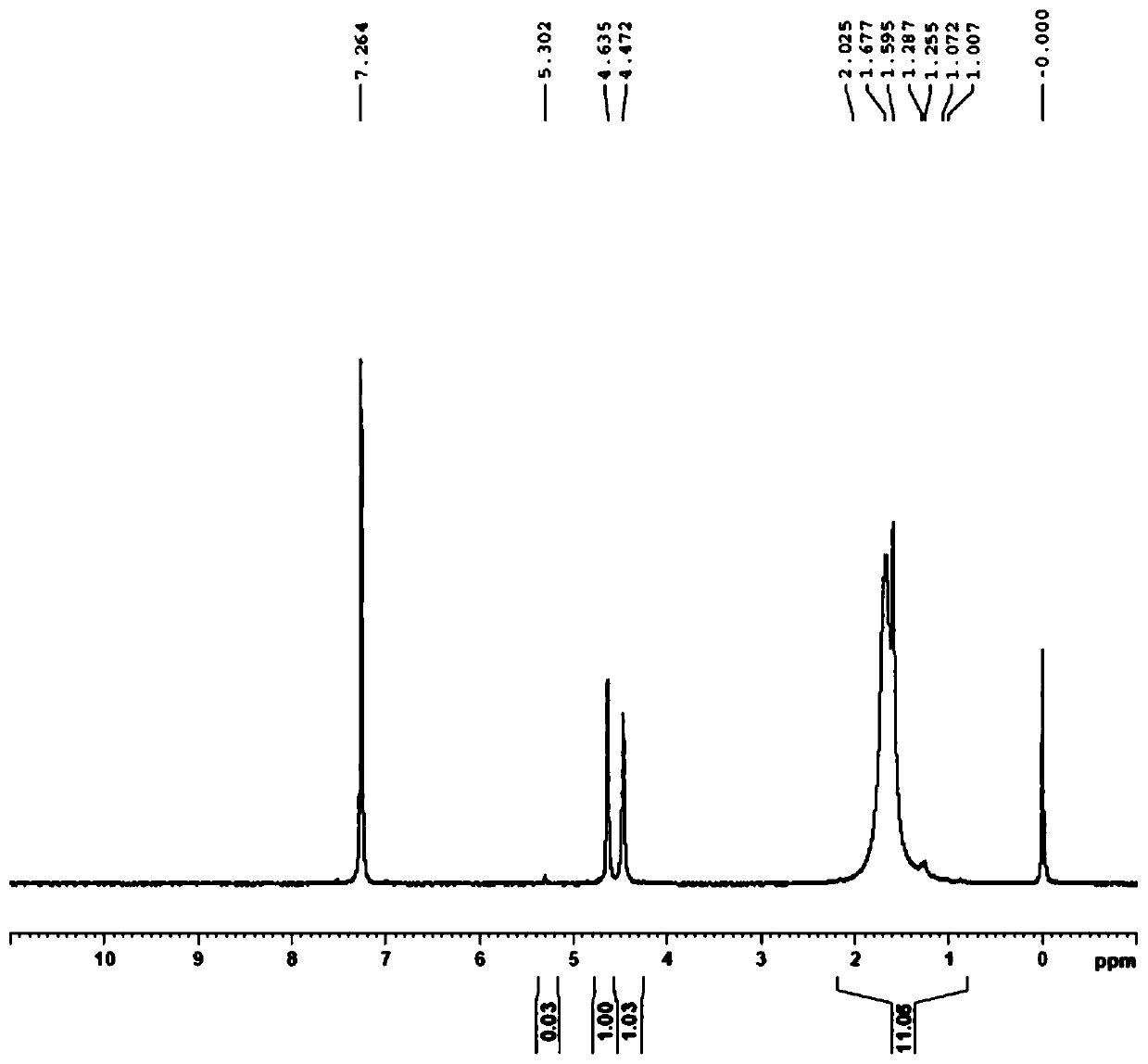
[0032] Dissolve palladium and palladium chloride in 20% dilute hydrochloric acid, pass the resulting palladium solution through a D301 anion exchange resin column (Langfang Kaiou), exchange the chloropalladate group on the resin, and 1,1'-bis( Di-tert-butylphosphine) ferrocene (DtBPF) ethanol solution flows through the resin to obtain a red suspension; the suspension is left to stand and then filtered, and the resin is washed with ethanol to separate the resin and the precipitate. Obtain 1,1'-bis(tert-butylphosphino)ferrocene palladium chloride, the productive rate is 82%, the palladium content is 15.48%, the elemental analysis carbon content: 46.74%, nuclear magnetic H-NMR sees attached figure 1 .
Embodiment 2
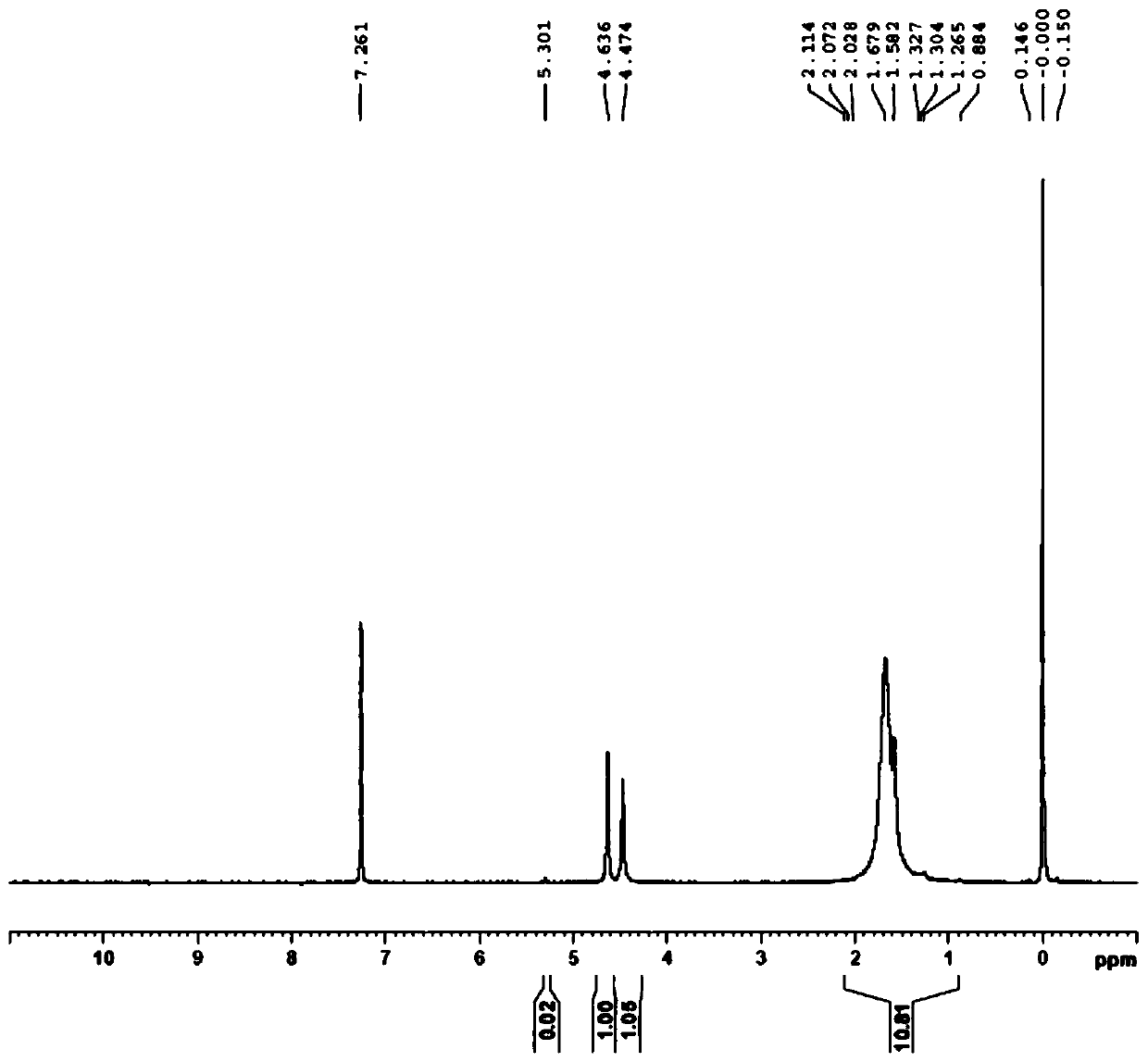
[0034] Dissolve palladium and palladium chloride in 20% dilute hydrochloric acid, pass the resulting palladium solution through a D301 anion exchange resin column (Langfang Kaiou), exchange the chloropalladate group on the resin, and 1,1'-bis( Di-tert-butylphosphine) ferrocene (DtBPF) solution, the solvent is ethanol and n-hexane, flow through the resin to obtain a red suspension; filter the suspension after standing, and use ethanol to wash the resin to separate the resin and precipitate. Obtain 1,1'-di(tert-butylphosphino)ferrocene palladium chloride, the yield is 87%, the palladium content is 15.38%, the elemental analysis carbon content: 48.09%, nuclear magnetic H-NMR sees attached figure 2 .
Embodiment 3
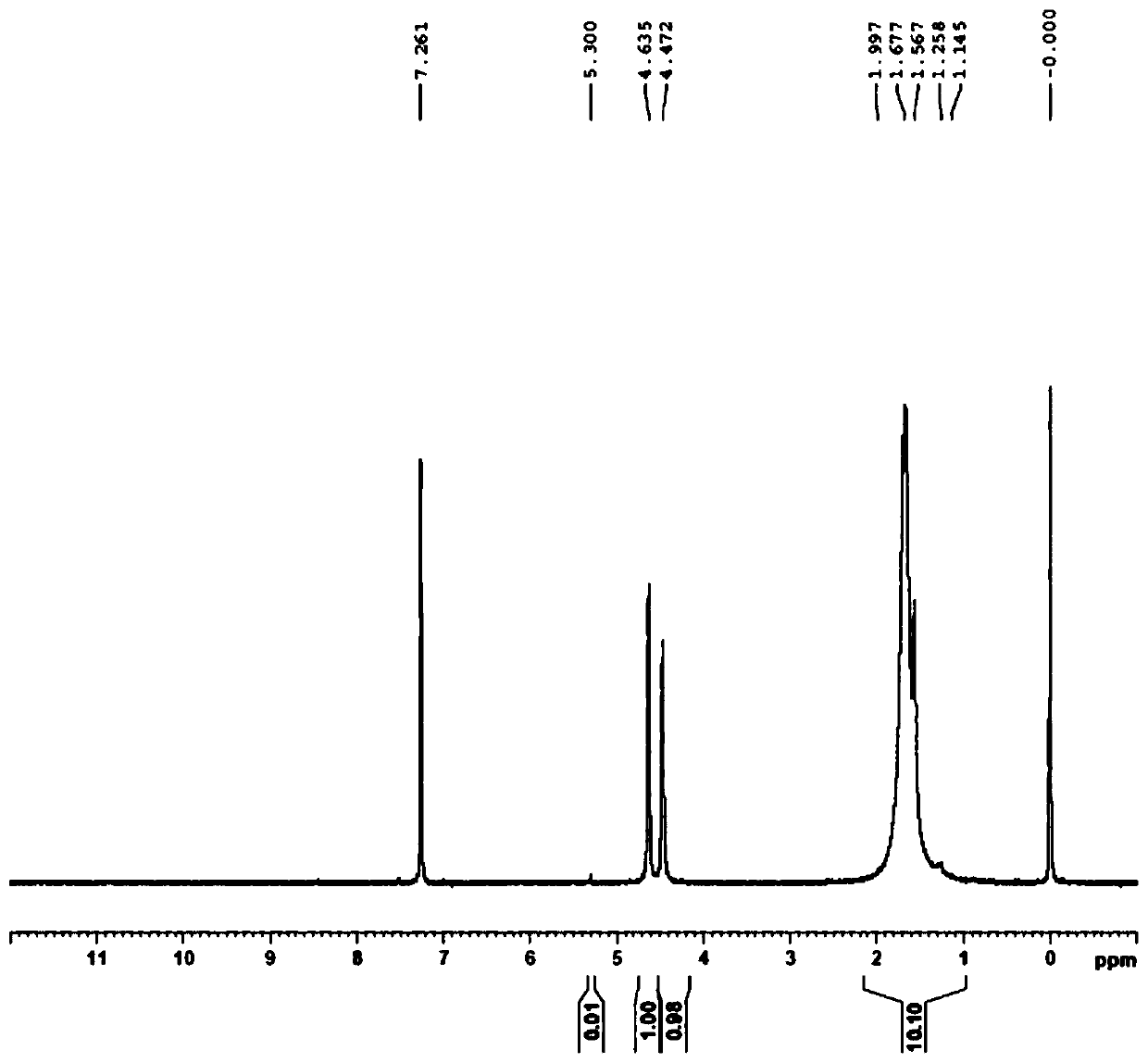
[0036] Dissolve palladium and palladium chloride in 18% dilute hydrochloric acid, pass the resulting palladium solution through a D301 anion exchange resin column (Langfang Kaiou), exchange the chloropalladate group on the resin, and 1,1'-bis( Di-tert-butylphosphine) ferrocene (DtBPF) tetrahydrofuran ethanol solution flows through the resin to obtain a red suspension; the suspension is left to stand and then filtered, and the resin is washed with ethanol to separate the resin and precipitate. Obtain 1,1'-bis(tert-butylphosphino)ferrocene palladium chloride, the yield is 91%, the palladium content is 15.37%, the elemental analysis carbon content: 47.84%, nuclear magnetic H-NMR sees attached image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com