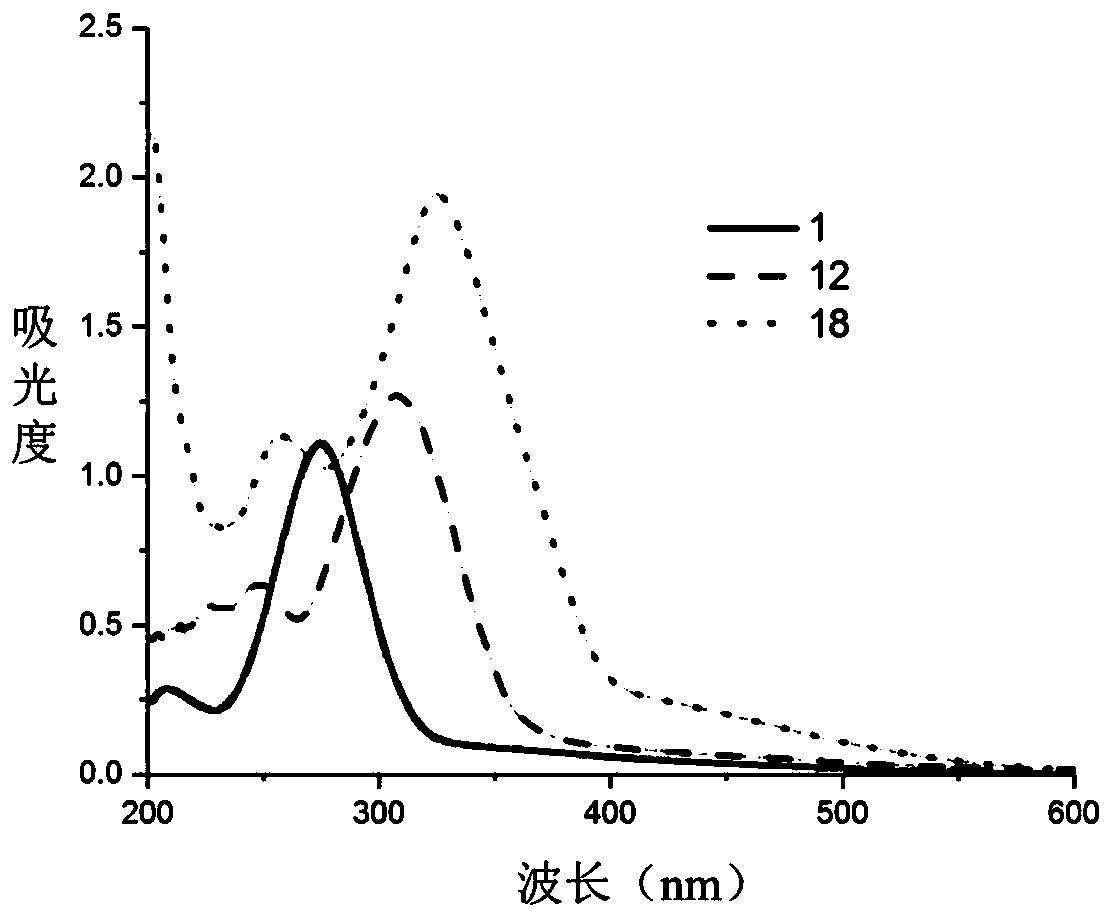
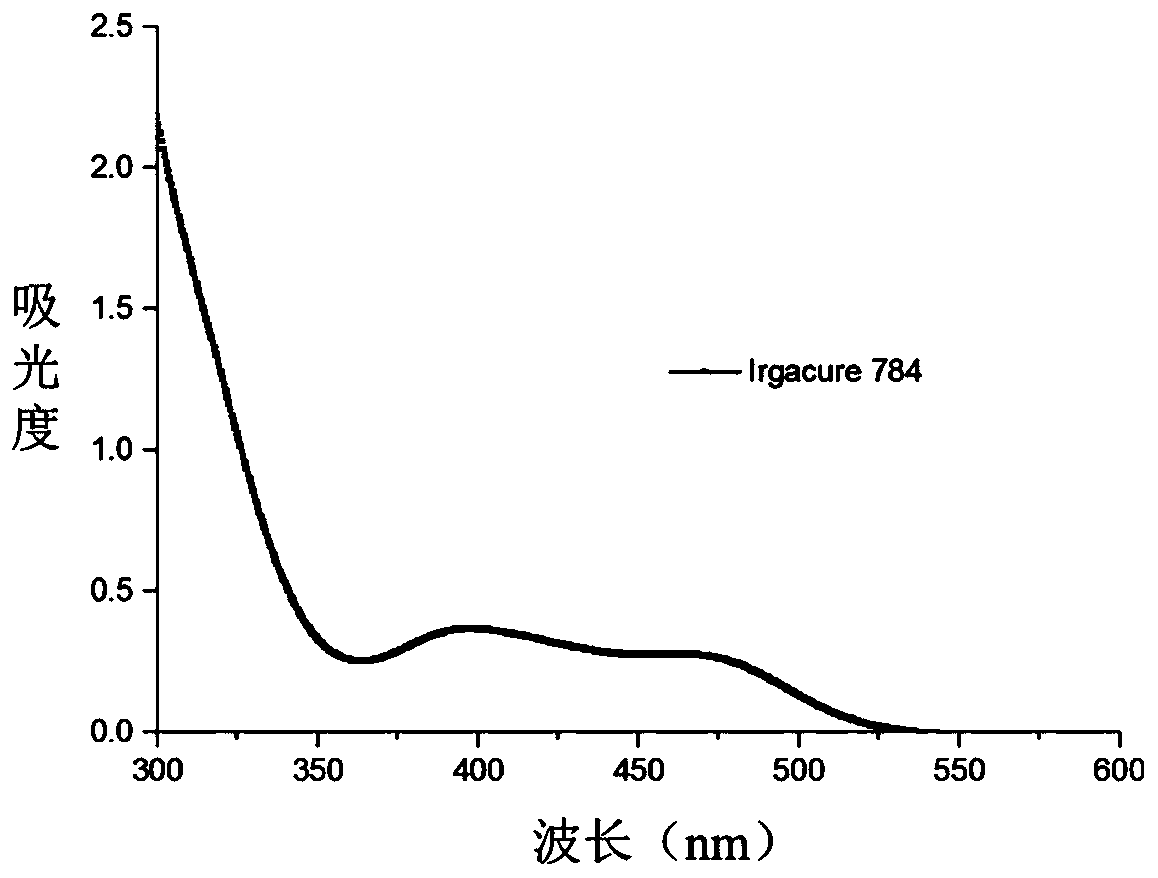
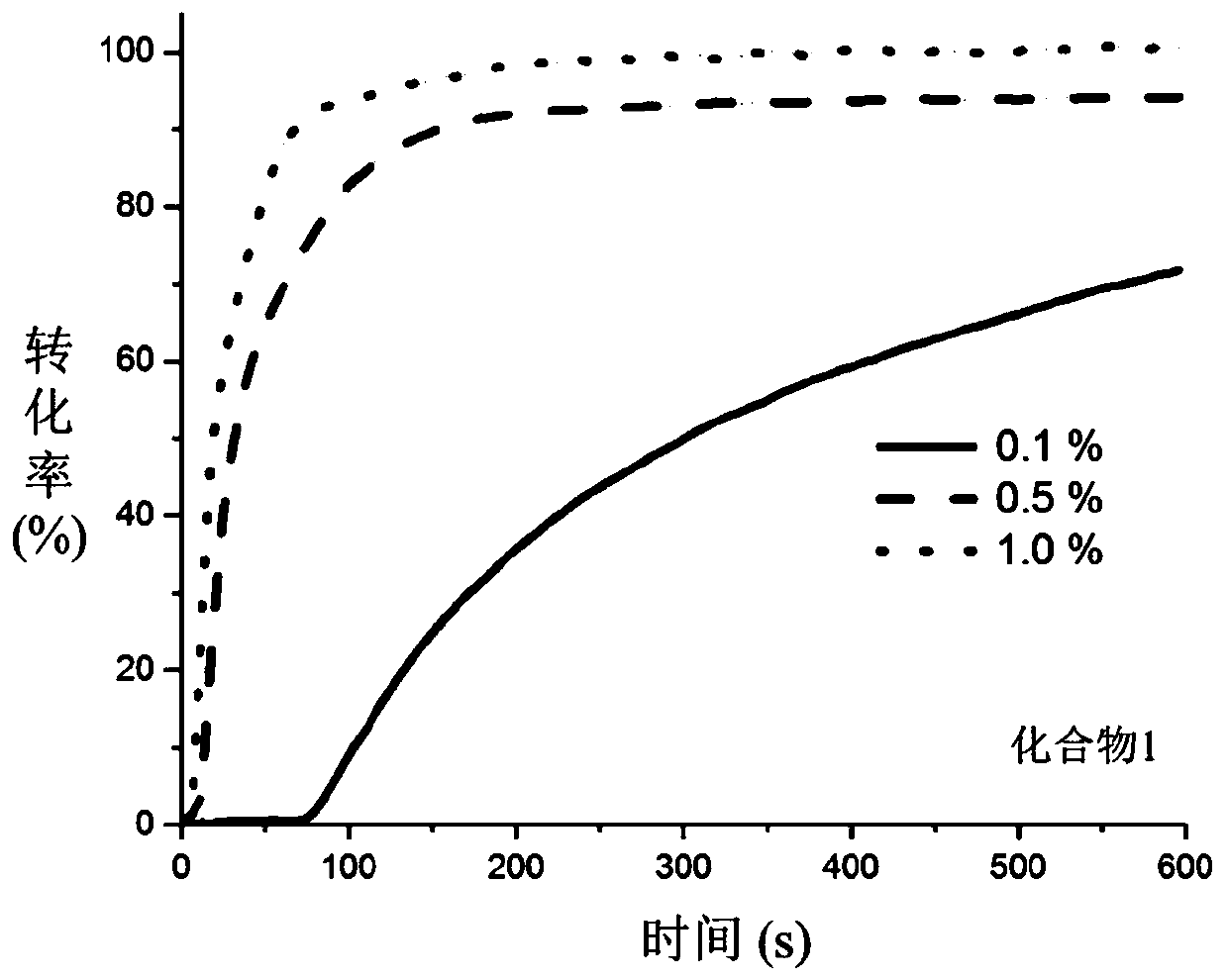
Beta-diketone cerium (IV) compound, preparation and application thereof
A compound, C1-C6 technology, applied in the field of preparation and its application as a photoinitiator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1: the preparation of compound 1
[0112]
[0113] The synthetic route of compound 1 is as follows:
[0114]
[0115] In a 100mL three-necked flask, the CeCl 3 ·7H 2 O (1.86 g, 5 mmol) was dissolved in 10 mL of ethanol. Acetylacetone (2.50 g, 25 mmol) was dissolved in 10 mL of ethanol in advance, and then added to the solution in the above-mentioned three-necked flask to obtain a pale yellow transparent reaction solution. After stirring for 10 minutes, 12.5 mL of 2M dilute ammonia water was added dropwise, and the reaction solution gradually turned orange and then reddish brown. The reaction was stirred at room temperature for 2 h, and most of the solvent was vacuum distilled out of the system after the reaction. A red-brown solid was obtained by filtration, washed with 10 mL of ethanol, and the washing was repeated twice, and vacuum-dried for 12 hours to obtain a red-brown powder with a yield of 2.23 g and a yield of 83.1%, which was identified as ...
Embodiment 2
[0117] Embodiment 2: the preparation of compound 12
[0118]
[0119] The synthetic route of compound 12 is as follows:
[0120]
[0121] In a 100mL three-necked flask, the CeCl 3 ·7H 2 O (1.86 g, 5 mmol) was dissolved in 10 mL of ethanol. Benzoylacetone (3.5 g, 22.5 mmol) was dissolved in 10 mL of ethanol in advance, and then added to the solution in the above-mentioned three-necked flask to obtain a pale yellow transparent reaction solution. After stirring for 10 min, 3.5 mL of triethylamine (25.2 mmol) was added dropwise, and the reaction solution gradually turned orange and then reddish brown. Stir the reaction at room temperature for 2h, then filter, and the filter residue is a reddish-brown solid. The obtained solid was washed with 5 mL of ethanol and dried in vacuo for 12 h to obtain a reddish-brown solid with a yield of 1.84 g and a yield of 46.9%, which was identified as compound 12.
[0122] 1 H NMR (400MHz, DMSO) δ7.88 (d, J = 6.8Hz, 8H), 7.58–7.19 (m, 1...
Embodiment 3
[0123] Embodiment 3: the preparation of compound 18
[0124]
[0125] The synthetic route of compound 18 is as follows:
[0126]
[0127] In a 100mL three-necked flask, Ce(NO 3 ) 3 ·6H 2 O (0.43 g, 1 mmol) was dissolved in 5 mL of absolute ethanol. Dibenzoylmethane (0.90 g, 4 mmol) was dissolved in 10 mL of absolute ethanol in advance, and then added to the solution in the above-mentioned three-necked flask to obtain a pale yellow transparent reaction solution. After stirring for 10 min, 4 mL of 1M dilute ammonia water was added dropwise, and the reaction solution turned reddish brown. Stir the reaction at room temperature for 2h, then filter, and the filter residue is a reddish-brown solid. The obtained solid was washed with 5 mL of absolute ethanol, and dried in vacuum for 12 h to obtain a reddish-brown solid with a yield of 0.99 g and a yield of 95.8%, which was identified as compound 18.
[0128] 1 H NMR (400MHz, CDCl 3 )δ8.04–7.99 (m,16H), 7.46–7.28 (m,24H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com