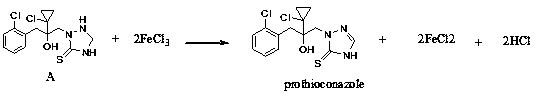
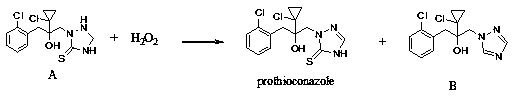
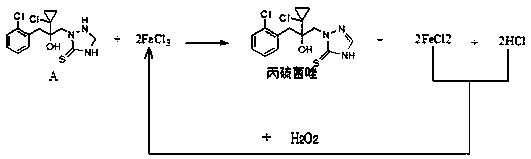
Synthetic method of prothioconazole
A technology of prothioconazole and synthesis method, which is applied in the field of synthesis of new broad-spectrum triazole thione fungicides, can solve the problems of strong oxidation ability, desulfurization impurities, etc., and achieve the effect of avoiding the three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 7.3g of intermediate A (95%, 20mmol), 20ml of ethanol and 0.066g (98%, 0.4mmol) of ferric chloride into the three-necked flask, cool to 20°C, and dropwise add 7.15g of hydrogen peroxide (10%, 21mmol) , the dropping time is 2 hours; after the dropwise addition, keep warm for 1 hour, add 50ml of water and 50ml of ethyl acetate, separate the liquids, extract the aqueous phase twice with 30ml of ethyl acetate, combine the organic phases, wash with 30ml of water three times, no Dry over sodium sulfate, concentrate under reduced pressure in vacuo to obtain 7.20 g of a light yellow solid, as determined by HPLC: the content of prothioconazole is 93.5%, the content of impurity B is 0.9%, and the yield is 97.6%.
Embodiment 2
[0031] Add 7.3g of intermediate A (95%, 20mmol), 20ml of tetrahydrofuran and 0.066g (98%, 0.4mmol) of ferric chloride into the three-necked flask, cool to 20°C, and dropwise add 7.15g of hydrogen peroxide (10%, 21mmol) , the dropping time is 2 hours; after the dropwise addition, keep warm for 1 hour, add 50ml of water and 50ml of ethyl acetate, separate the liquids, extract the aqueous phase twice with 30ml of ethyl acetate, combine the organic phases, wash with 30ml of water three times, no Dry over sodium sulfate, concentrate under reduced pressure in vacuo to obtain 7.17 g of a light yellow solid, as determined by HPLC: the content of prothioconazole is 92.7%, the content of impurity B is 0.8%, and the yield is 96.4%.
Embodiment 3
[0033] Add 7.3g of intermediate A (95%, 20mmol), 20ml of tetrahydrofuran and 0.099g (98%, 0.6mmol) of ferric trichloride into the three-necked flask, cool to 20°C, and dropwise add 7.15g of hydrogen peroxide (10%, 21mmol) , the dropping time is 2 hours; after the dropwise addition, keep warm for 1 hour, add 50ml of water and 50ml of ethyl acetate, separate the liquids, extract the aqueous phase twice with 30ml of ethyl acetate, combine the organic phases, wash with 30ml of water three times, no Dry over sodium sulfate, concentrate under reduced pressure in vacuo to obtain 7.06 g of a light yellow solid, as determined by HPLC: the content of prothioconazole is 93.2%, the content of impurity B is 0.6%, and the yield is 95.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com