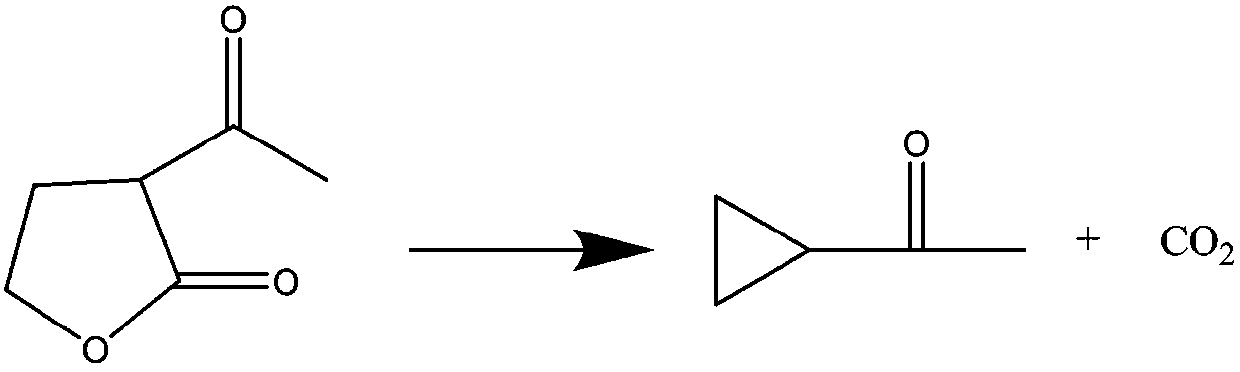
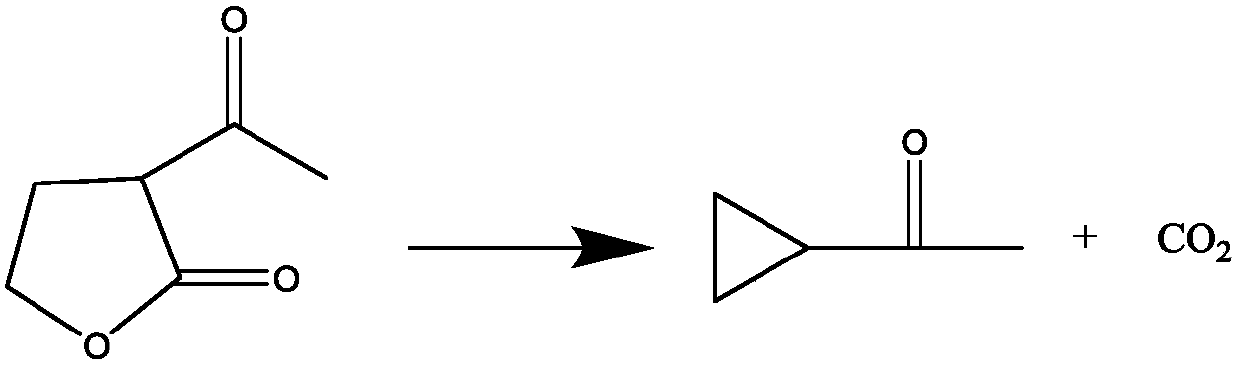
Novel preparation method of cyclopropyl methyl ketone
A technology of cyclopropane ketone and methyl imidazolium ion, which is applied in the preparation of heterocyclic compounds, organic chemistry and other directions, can solve the problems of low reaction yield, high reaction temperature, low product content, etc., and achieves improved operation efficiency and reaction selection. High performance and cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) In the microwave reactor, add 200 g of 1-butyl-3-methylimidazolium chloride salt ionic liquid, start stirring, start microwave heating, and raise the temperature to 140°C;
[0024] 2) At a temperature of 140°C, slowly and continuously add α-acetyl-γ-butyrolactone, microwaves promote the catalyzed cracking reaction of the ionic liquid, and continuously extract the cracked product cyclopropanone through the rectification tower. The raw material addition rate was 10 g / min.
[0025] 3) After the raw material feeding is completed, the distillation is continued for 1 hour to collect the complete product cyclopropanone. After the reaction, the obtained ionic liquid can be directly used for the preparation of the next batch of cyclopropanone products without any treatment.
[0026] The content of the collected cyclopropanone was 99.10%, and the total molar yield of the reaction was 98.2%.
Embodiment 2
[0028] 1) In the microwave reactor, add 200 g of 1-ethyl-3-methylimidazolium bromide salt ionic liquid, start stirring, start microwave heating, and raise the temperature to 100°C;
[0029] 2) At a temperature of 100°C, α-acetyl-γ-butyrolactone is added slowly and continuously, microwaves promote the catalyzed cracking reaction of the ionic liquid, and at the same time, the cracked product cyclopropanone is continuously extracted through the rectification tower. The feed rate was 1.0 g / min.
[0030] 3) After the raw material feeding is completed, continue to distill and extract for 2 hours, and collect the complete product cyclopropanone. After the reaction, the obtained ionic liquid can be directly used for the preparation of the next batch of cyclopropanone products without any treatment.
[0031] The content of the collected cyclopropanone was 99.5%, and the total molar yield of the reaction was 98.9%.
Embodiment 3
[0033] 1) In the microwave reactor, add 200 g of 1-butyl-3-methylimidazolium iodide salt ionic liquid, start stirring, start microwave heating, and raise the temperature to 120°C;
[0034] 2) At a temperature of 120°C, α-acetyl-γ-butyrolactone is added slowly and continuously, microwaves promote the catalytic cracking reaction of the ionic liquid, and at the same time, the cracking product cyclopropanone is continuously extracted through the rectification tower. The feed rate was 2.0 g / min.
[0035] 3) After the raw material feeding is completed, the distillation is continued for 1 hour to collect the complete product cyclopropanone. After the reaction, the obtained ionic liquid can be directly used for the preparation of the next batch of cyclopropanone products without any treatment.
[0036] The content of the collected cyclopropanone was 99.70%, and the total molar yield of the reaction was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com