A kind of preparation method of isoxazole compound and its intermediate
A compound and isoxazole technology, applied in the field of organic synthesis, can solve the problems of dangerous post-processing process, many impurities and high cost, and achieve the effects of avoiding safety and three waste problems, high product purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
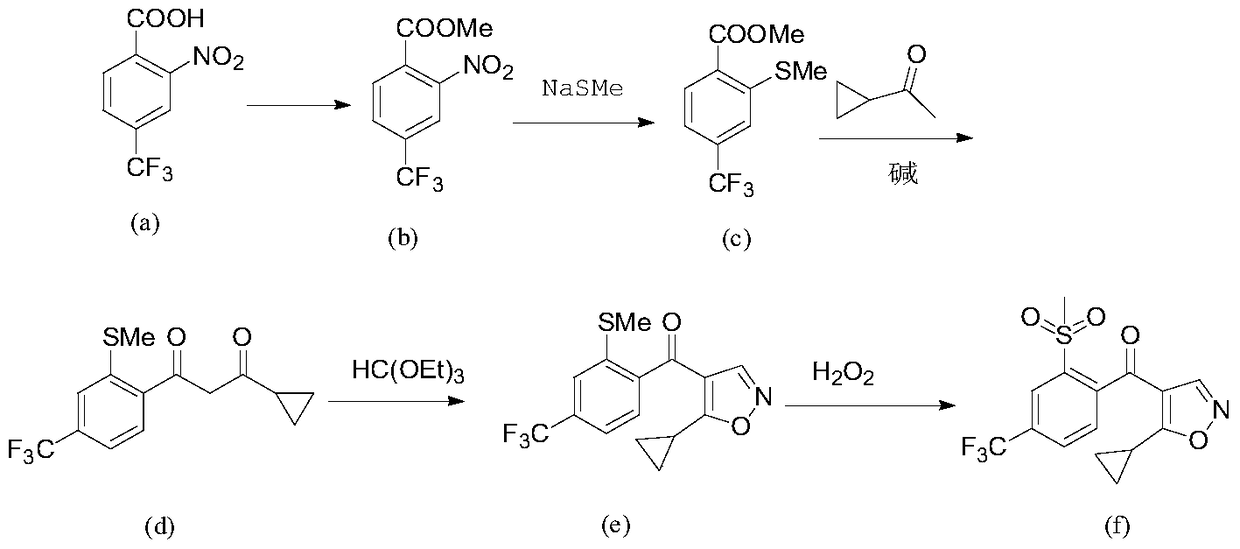
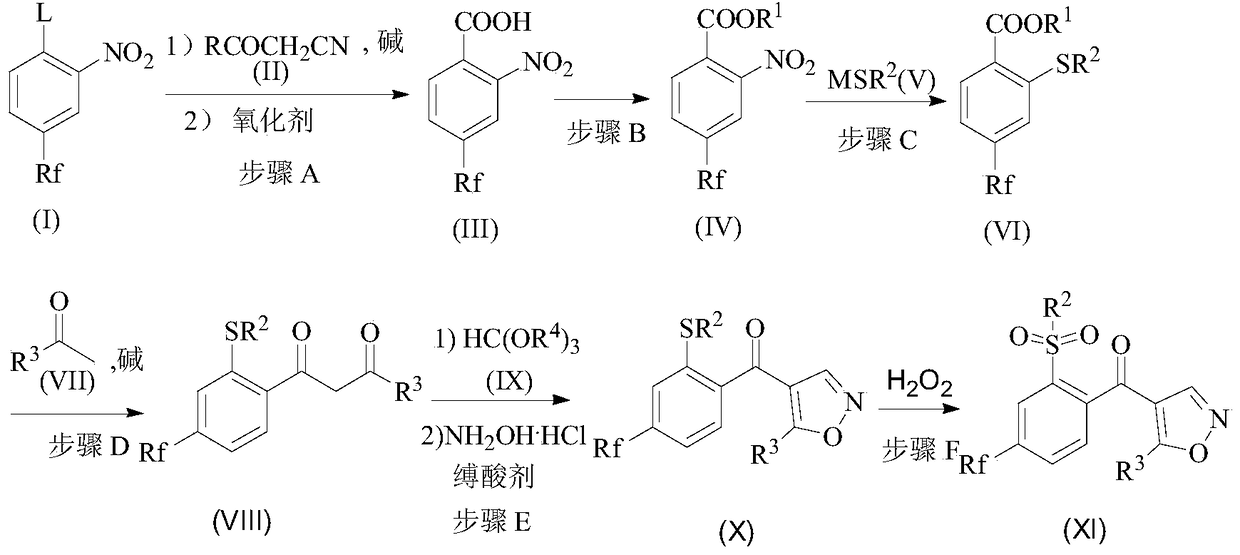
[0035] A preparation method of isoxazole compound includes the following steps:
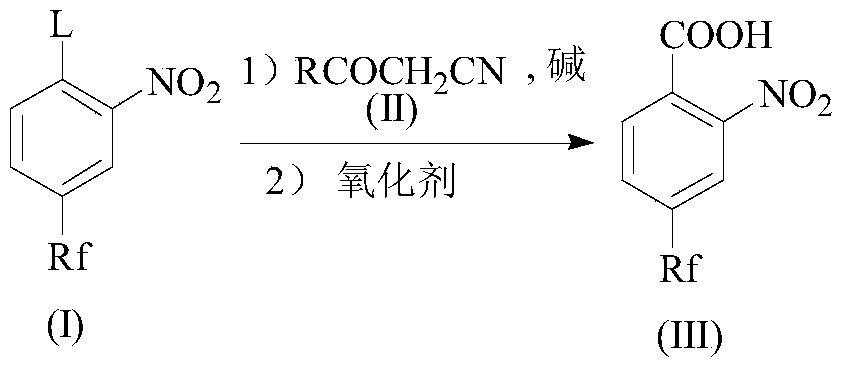
[0036] Step A, after the compound (I) reacts with the compound (II) at a certain temperature in a solvent and the presence of a base, the reaction is continued with an oxidizing agent to obtain an intermediate compound (III);
[0037] Step B: After the compound (III) is reacted with an acid chlorination reagent, it is reacted with an alcohol at a certain temperature to obtain compound (IV), or compound (III) is reacted with an alkylating reagent at a certain temperature under the action of a base to obtain compound ( IV);
[0038] Step C: In a solvent, compound (IV) is reacted with sulfide (V) at a certain temperature to obtain compound (VI);
[0039] Step D, in the presence of a solvent and a base, compound (VI) and methyl ketone (VII) are condensed at a certain temperature to obtain compound (VIII);
[0040] Step E: In the solvent, after the compound (VIII) and the orthoformate (IX) undergo an etherific...
Embodiment 1
[0060] Example 1: Preparation of 4-chloro-3-nitrobenzotrifluoride
[0061] In a 1000mL three-necked flask, add 400g p-chlorobenzotrifluoride, add dropwise a mixed acid of 273g 98% sulfuric acid and 190g 98% nitric acid at 30°C, continue the reaction for 4 hours after the addition is complete, separate the organic layer, and wash the organic layer with water Two times, 480 g of 4-chloro-3-nitrobenzotrifluoride was obtained.
Embodiment 2
[0062] Example 2: Preparation of 2-nitro-4-trifluoromethylbenzoic acid
[0063] In a 1000 mL three-necked flask equipped with a thermometer, 500 g of DMF, 180 g of potassium carbonate, and 78 g of ethyl cyanoacetate were added. Add 150 g of 4-chloro-3-nitrobenzotrifluoride to control the temperature of the reactant within 50° C., after the addition is complete, continue to stir and react for 1 hour. Drop 230g 35% H 2 O 2 After the dropwise addition is completed, the reaction is continued for 2 hours, the hydrogen peroxide is quenched, the solution is removed, water is added, acidified with hydrochloric acid, filtered, washed with water, and dried to obtain 149 g of 2-nitro-4-trifluoromethylbenzoic acid. 1 H-NMRδppm(DMSO-d 6 ): 14.39 (br, 1H), 8.46 (s, 1H), 8.21 (d, J=8.0 Hz, 1H), 8.09 (d, J=8.0 Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com