A method for recovering sulfuric acid from waste sulfuric acid containing hydrogen peroxide
A technology of hydrogen peroxide and waste sulfuric acid, which is applied in the electrolysis process, electrolysis components, electrodes, etc., can solve the problems of unable to realize the recovery of sulfuric acid and the introduction of impurities, and achieve the effects of low cost, easy control of the reaction, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
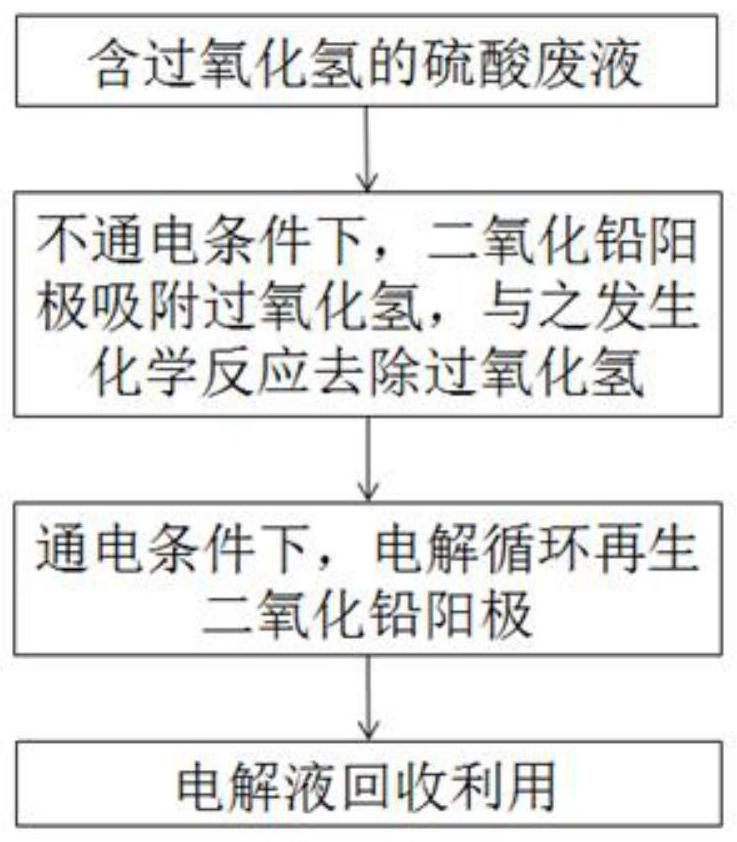
[0031] A recovery treatment method for waste sulfuric acid, comprising the following steps:
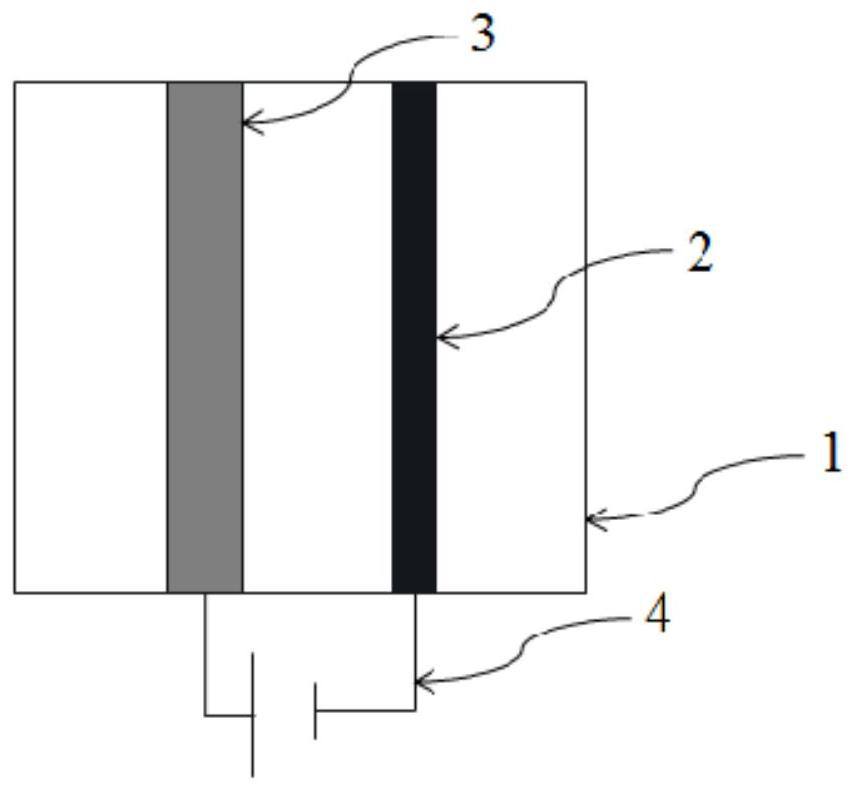
[0032] Step A: collect the sulfuric acid waste liquid containing hydrogen peroxide, pass it into the electrolytic cell, and under the condition of no electricity, the anode performs the reaction of absorbing hydrogen peroxide;
[0033] Step B: under energized conditions, use the solution in step A as the electrolyte to perform electrolysis in the electrolytic cell to regenerate the lead dioxide anode;
[0034] Step C: Recycling the electrolytic solution that has been electrolyzed in Step B.
[0035] The concentration of sulfuric acid in the original waste liquid in step A is 0.5 mol / L, and the concentration of hydrogen peroxide is about 10.65 g / L.
[0036] The anode of the electrolytic cell in the step B is a titanium-based lead dioxide electrode, and the cathode is a carbon electrode; the current intensity is 5mA / cm 2 . After the electrolysis in the electrolyzer is completed, the ...
Embodiment 2
[0038] A method for reclaiming sulfuric acid from waste sulfuric acid containing hydrogen peroxide, comprising the following steps:
[0039] Step A: collect the sulfuric acid waste liquid containing hydrogen peroxide, pass it into the electrolytic cell, and under the condition of no electricity, the anode performs the reaction of absorbing hydrogen peroxide;
[0040] Step B: under energized conditions, use the solution in step A as the electrolyte to perform electrolysis in the electrolytic cell to regenerate the lead dioxide anode;
[0041] Step C: Recycling the electrolytic solution that has been electrolyzed in step B.
[0042] The concentration of sulfuric acid in the original waste liquid in step A is 0.5 mol / L, and the concentration of hydrogen peroxide is about 11.34 g / L.
[0043] The anode of the electrolytic cell in the step B is a titanium-based lead dioxide electrode, and the cathode is a carbon electrode; the current intensity is 10mA / cm 2 . After the electrolys...
Embodiment 3
[0045] A method for reclaiming sulfuric acid from waste sulfuric acid containing hydrogen peroxide, comprising the following steps:
[0046] Step A: collect the sulfuric acid waste liquid containing hydrogen peroxide, pass it into the electrolytic cell, and under the condition of no electricity, the anode performs the reaction of absorbing hydrogen peroxide;
[0047] Step B: under energized conditions, use the solution in step A as the electrolyte to perform electrolysis in the electrolytic cell to regenerate the lead dioxide anode;
[0048] Step C: Recycling the electrolytic solution that has been electrolyzed in step B.
[0049] The concentration of sulfuric acid in the original waste liquid in step A is 0.5 mol / L, and the concentration of hydrogen peroxide is about 10.51 g / L.
[0050] The anode of the electrolytic cell in the step B is a titanium-based lead dioxide electrode, and the cathode is a carbon electrode; the current intensity is 15mA / cm 2 . After the electrolys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com