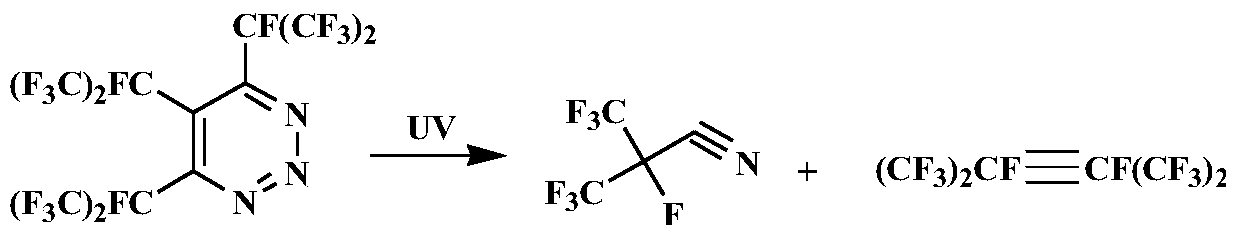
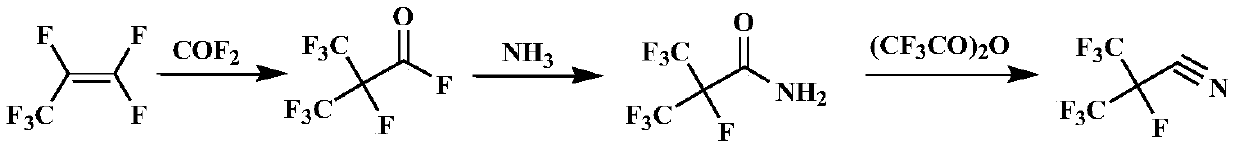
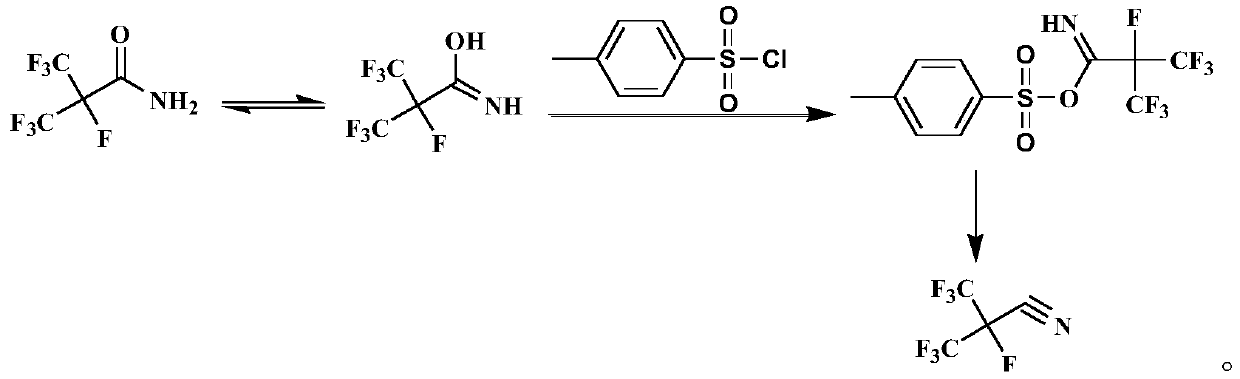
Preparation method of heptafluoroisobutyronitrile
A technology for heptafluoroisobutyronitrile and heptafluoroisobutyramide is applied in the field of preparation of perfluoroisobutyronitrile, can solve the problems of low yield, unfavorable industrial scale up, high cost, and achieves high reaction yield and easy industrial production , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The volume of the reaction kettle is 10L, equipped with stirring, thermometer and condenser, and connected with gas cooling and absorption device. Add 3.00 kg of methyl tert-butyl ether, 2.10 kg of p-toluenesulfonyl chloride, and 2.13 kg of heptafluoroisobutyramide into the reaction kettle, and cool down to -10°C after the addition. Then start to add 1.2 kg of triethylamine dropwise, control the reaction temperature at -10 to 0°C, and keep the temperature at -10°C for 2.0 hours after the dropwise addition. After the reaction, the temperature was raised to 30° C. and kept for 4.0 hours. During the process, the gas escaped from the system was cooled to make it liquefied, and a total of 1.92 kg of heptafluoroisobutyronitrile was obtained with a purity of 99.6% and a yield of 98.1%.
Embodiment 2
[0040] The volume of the reaction kettle is 10L, equipped with stirring, thermometer and condenser, and connected with gas cooling and absorption device. Add 3.00 kg of methyl tert-butyl ether, 2.10 kg of p-toluenesulfonyl chloride, and 2.13 kg of heptafluoroisobutyramide into the reaction kettle, and cool down to -10°C after the addition. Then start to add 1.1 kg of aniline dropwise, control the reaction temperature at -10 to 0°C, and keep the temperature at -10°C for 2.0 hours after the dropwise addition. After the reaction, the temperature was raised to 30° C. and kept for 4.0 hours. During the process, the gas escaped from the system was cooled and liquefied to obtain 1.77 kg of heptafluoroisobutyronitrile with a purity of 99.2% and a yield of 90.4%.
Embodiment 3
[0042] The volume of the reaction kettle is 10L, equipped with stirring, thermometer and condenser, and connected with gas cooling and absorption device. Add 3.00 kg of methyl tert-butyl ether, 2.10 kg of p-toluenesulfonyl chloride, and 2.13 kg of heptafluoroisobutyramide into the reaction kettle, and cool down to -10°C after the addition. Then start to add 1.0 kg of cyclohexylamine dropwise, control the reaction temperature at -10 to 0°C, and keep the temperature at -10°C for 2.0 hours after the dropwise addition. After the reaction, the temperature was raised to 30° C. and kept for 4.0 hours. During the process, the gas escaped from the system was cooled and liquefied to obtain 1.83 kg of heptafluoroisobutyronitrile with a purity of 99.6% and a yield of 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com